Novel diarylheptanoids as inhibitors of TNF-α production
Sameer Dhurua, Dilip Bhedia, Dnyaneshwar Gophanea, Kiran Hirbhagata, Vijaya Nadara, Dattatray Morea, Sapna Parikha, Roda Dalala, Lyle C. Fonsecaa, Firuza Kharasa, Prashant Y. Vadnala, Ram A. Vishwakarmaa, H. Sivaramakrishnana*
aDepartment of Medicinal Chemistry, Piramal Life Sciences Limited, 1 Nirlon Complex, Off Western Express Highway, Goregaon (E), Mumbai 400 063, India
bDepartment of Pharmacology, Piramal Life Sciences Limited, 1 Nirlon Complex, Off Western Express Highway, Goregaon (E), Mumbai 400 063, India
Bioorg. Med. Chem. Lett. 21 (2011) 3784–3787
[Link: http://pubs.rsc.org/en/content/articlelanding/2013/cc/c2cc36389e#!divAbstract]
Graphical abstract
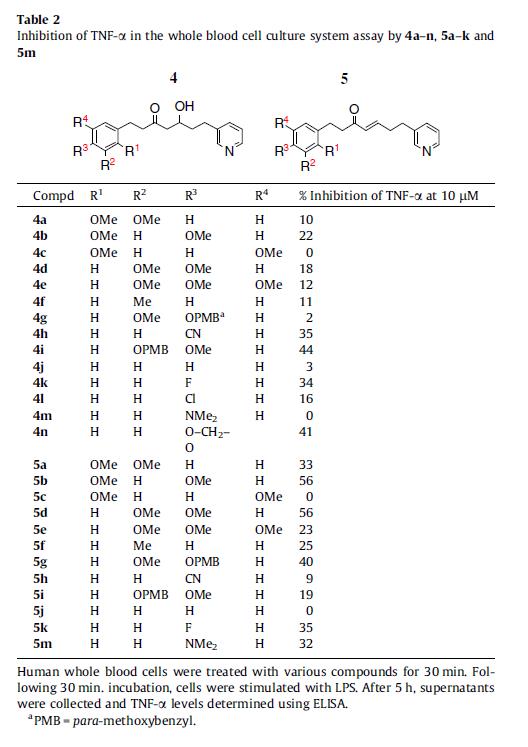
Synthesis and anti-inflammatory activity of novel diarylheptanoids [5-hydroxy-1-phenyl-7-(pyridin-3-yl)-heptan-3-ones and 1-phenyl-7-(pyridin-3-yl)hept-4-en-3-ones] as inhibitors of tumor necrosis factor-α (TNF-α production is described in the present article. The key reactions involve the formation of a β-hydroxyketone by the reaction of substituted 4-phenyl butan-2-ones with pyridine-3-carboxaldehyde in presence of LDA and the subsequent dehydration of the same to obtain the α,β-unsaturated ketones. Compounds 4i, 5b, 5d, and 5g significantly inhibit lipopolysaccharide (LPS)-induced TNF-α production from human peripheral blood mononuclear cells in a dose-dependent manner. Of note, the in vitro TNF-α inhibition potential of 5b and 5d is comparable to that of curcumin (a naturally occurring diarylheptanoid). Most importantly, oral administration of 4i, 5b, 5d, and 5g (each at 100 mg/kg) but not curcumin (at 100 mg/kg) significantly inhibits LPS-induced TNF-α production in BALB/c mice. Collectively, our findings suggest that these compounds may have potential therapeutic implications for TNF-α-mediated auto-immune/inflammatory disorders.
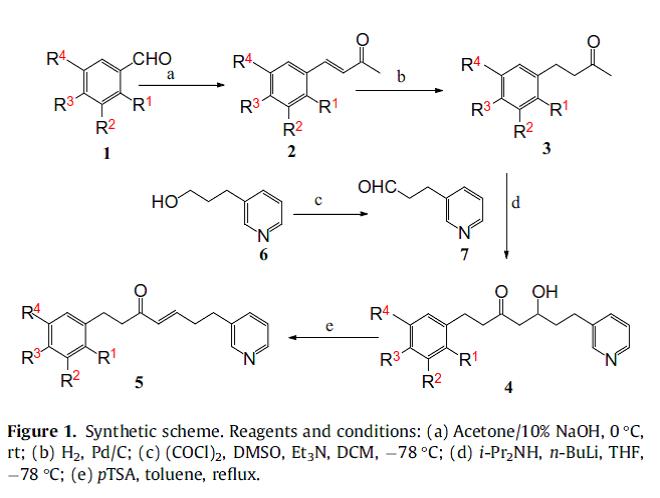
Scheme 1. Synthetic scheme
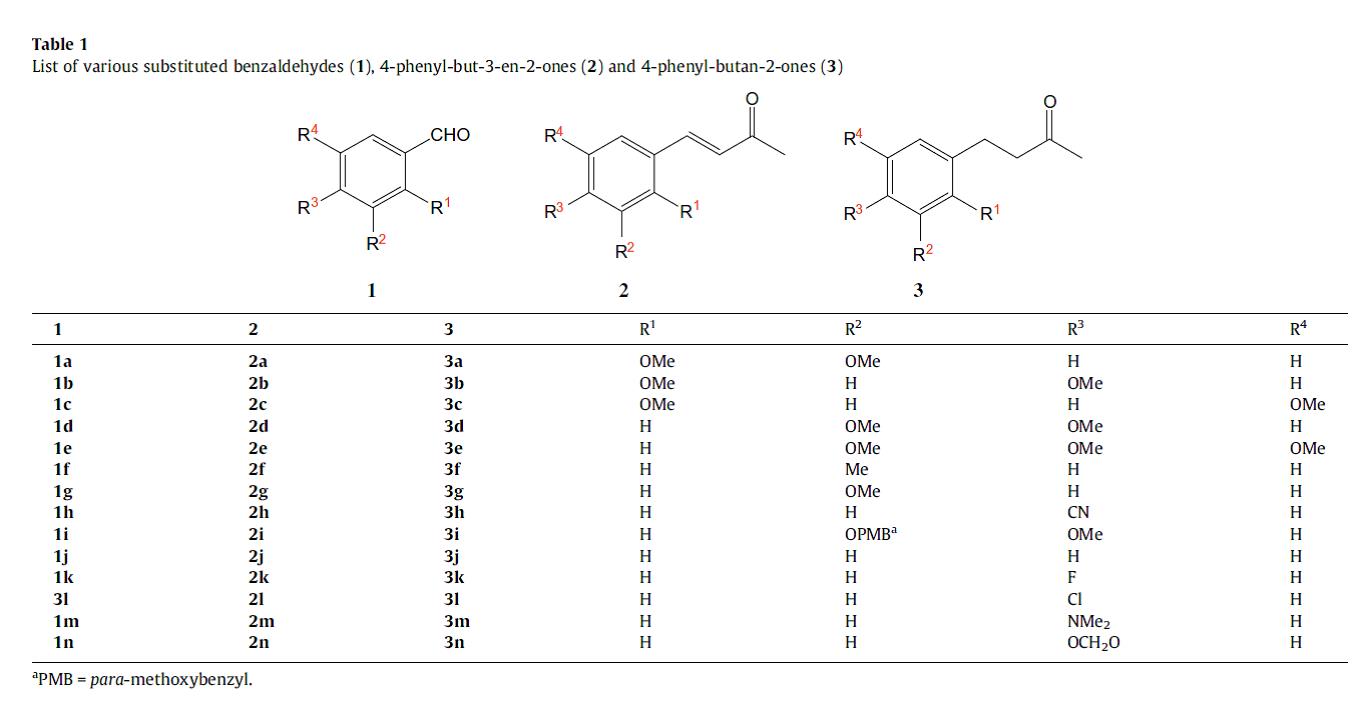
Table 1.
Table 2.
Highlights
- Designed and synthesized a novel series of diarylheptanoids.
- Compounds 4i, 5b, 5d, and 5g significantly inhibit in vitro TNF-α production from human cells.
- Oral administration of these compounds significantly inhibits TNF-α production in mice.
- These compounds may have potential therapeutic implications for TNF- α -mediated auto-immune/inflammatory diseases.
ABOUT GUEST BLOGGER
Dr. Dnyaneshwar B. Gophane, Ph. D.
Post doc fellow at Purdue university and university of Iceland
Email, gophane@gmail.com
Dr. Dnyaneshwar B. Gophane completed his B.Sc. (Chemistry) at Anand college of science, Pathardi (Ahmednagar, Maharashtra, India) in 2000 and M.Sc. (Organic Chemistry) at Department of Chemistry, University of Pune (India) in 2003. From 2003 to 2008, he worked in research and development departments of pharmaceutical companies like Dr. Reddy’s Laboratories and Nicholas Piramal India Limited, where he involved in synthesizing novel organic compounds for in vitro and in vivo screening and optimizing process for drug molecule syntheses. In 2008, Dnyaneshwar joined Prof. Sigurdsson’s laboratory for his Ph.D. study at the University of Iceland. His Ph.D. thesis mainly describes syntheses of nitroxide spin-labeled and fluorescent nucleosides and their incorporation into DNA and RNA using phosphoramidite chemistry. These modified nucleosides are useful probes for studying the structure and dynamics of nucleic acids by EPR and fluorescence spectroscopies. In 2014, after finishing his Ph.D., he worked as post doc fellow in same laboratory and mainly worked on spin labelling of RNA. At the university of Purdue in his second post doc, he was totally dedicated to syntheses of small molecules for anti-cancer activity and modification of cyclic dinucleotides for antibacterial activity. During his research experience, he has authored 8 international publications in peer reviewed journals like Chemical Communications, Chemistry- A European Journal, Journal of organic chemistry and Organic and Biomolecular Chemistry.