An efficient synthetic approach for the synthesis of α-pyrones via Baeyer–Villiger-type oxidation of α-iodocyclopentenones through a catalyst- and additive-free system using air as an environmentally benign oxidant is described. The reaction exhibits excellent functional group compatibility and provides a simple and efficient protocol for the construction of highly functionalized α-pyrones under mild reaction conditions.
Catalyst- and Additive-Free Baeyer−Villiger-type Oxidation of α-Iodocyclopentenones to α-Pyrones: Using Air as the Oxidant
Catalyst- and Additive-Free Baeyer−Villiger-type Oxidation of α-Iodocyclopentenones to α-Pyrones: Using Air as the Oxidant
Abstract
An efficient synthetic approach for the synthesis of α-pyrones via Baeyer−Villiger-type oxidation of α-iodocyclopentenones through a catalyst- and additive-free system using air as an environmentally benign oxidant is described. The reaction exhibits excellent functional group compatibility and provides a simple and efficient protocol for the construction of highly functionalized α-pyrones under mild reaction conditions.
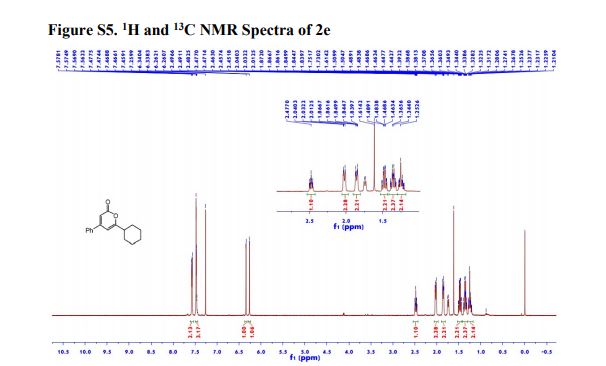
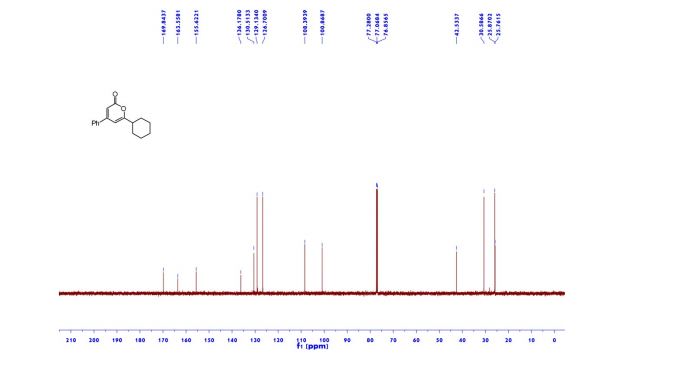
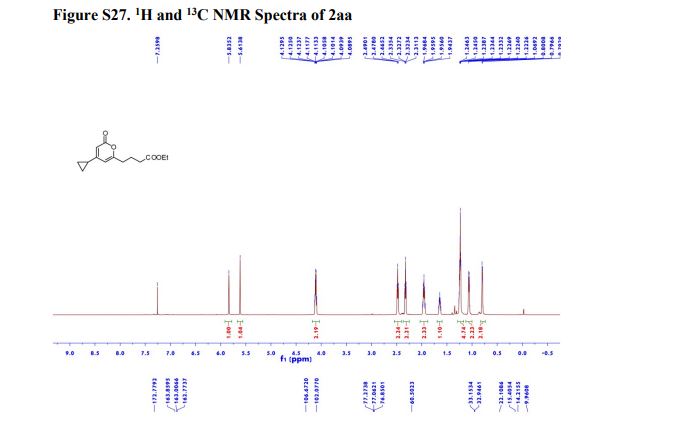
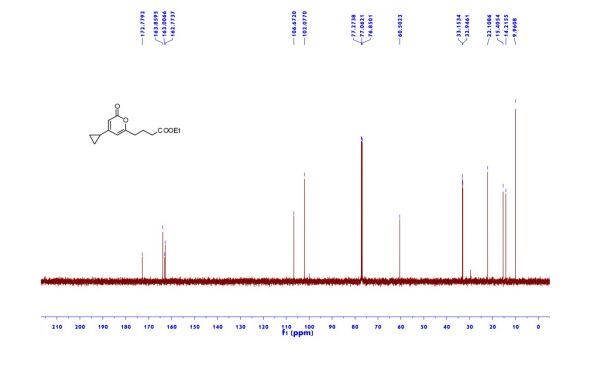
Ethyl 4-(4-cyclopropyl-2-oxo-2H-pyran-6-yl)butanoate (2aa) Product 2aa was obtained as yellow oil in 50% yield (38 mg) following the general procedure; 1H NMR (600 MHz, CDCl3) δ 5.84 (s, 1H), 5.61 (s, 1H), 4.13-4.09 (m, 2H), 2.48 (t, J = 7.3 Hz, 2H), 2.33 (td, J = 7.3, 2.3 Hz, 2H), 1.97-1.94 (m, 2H), 1.66-1.63 (m, 1H), 1.26-1.22 (m, 3H), 1.07-1.05 (m, 2H), 0.80-0.79 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 172.8, 163.9, 163.0, 162.8, 106.7, 102.1, 60.5, 33.2, 33.0, 22.1, 15.4, 14.2, 10.0; HRMS (ESI) calcd. for C14H18O4Na [M+Na]+ : 273.1097, found: 273.1101
////////////
http://www.rsc.org/suppdata/c9/gc/c9gc02725d/c9gc02725d1.pdf
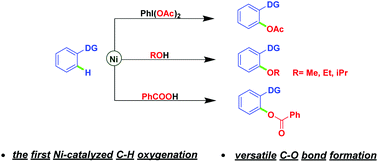
Nickel-catalyzed regioselective C–H oxygenation: new routes for versatile C–O bond formation
Nickel-catalyzed regioselective C–H oxygenation: new routes for versatile C–O bond formation
DOI: 10.1039/C8QO01274A, Research Article
Nickel-catalyzed regioselective C–H oxygenation reactions of chelating arenes using iodobenzene diacetate, alcohols, and benzoic acids respectively as attacking reagents have been developed for the first time.
To cite this article before page numbers are assigned, use the DOI form of citation above.
Abstract
Nickel-catalyzed regioselective C–H oxygenation reactions of chelating arenes using iodobenzene diacetate, alcohols, and benzoic acids respectively as attacking reagents have been developed for the first time. Simplicity of operation, broad range of functional group tolerance, use of cheap transition metal nickel, and avoiding extraneous directing groups are the key features, thus providing an important complement to C–H oxygenation reactions and expanding the field of nickel-catalyzed C–H functionalizations. Explorations of mechanistic details are also described.
Nickel-catalyzed regioselective C–H oxygenation: new routes for versatile C–O bond formation
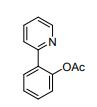
2-(pyridin-2-yl)phenyl acetate (2a)
Formula: C13H11NO2 Mass: 213
To a mixture of 2-phenylpyridine (77.5 mg, 0.5 mmol) 1a, Ni(acac)2 (25.7 mg, 0.1 mmol, 20 mol %), ligand MePh2P (20.0 mg, 0.1 mmol, 20 mol %), and PhI(OAc)2 (483.2 mg, 0.75 mmol, 1.5 equiv) in a reaction tube was added solvent (CH3CN=2.0 mL). The reaction mixture was stirred at 115 °C for 24 h in air. Following the general procedure, 2a was purified by column chromatography on silica gel (petroleum ether: ethyl acetate = 5:1) as a white solid (80.9 mg, 76%).
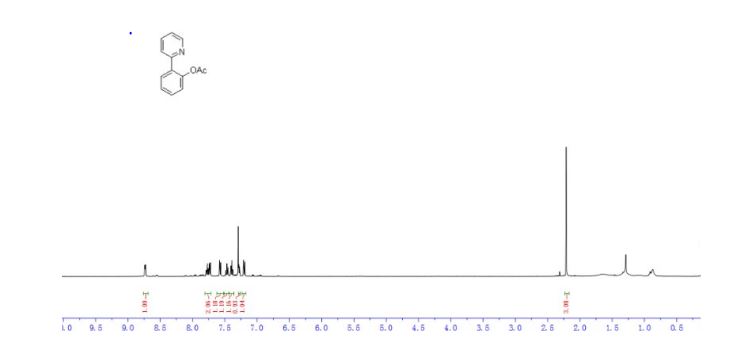
1H NMR (500 MHz, Chloroform-d) δ 8.8 – 8.7 (m, 1H), 7.8 – 7.7 (m, 2H), 7.6 (dd, J = 7.9, 1.1 Hz, 1H), 7.5 (td, J = 7.7, 1.7 Hz, 1H), 7.4 (td, J = 7.5, 1.2 Hz, 1H), 7.3 – 7.3 (m, 1H), 7.2 (dd, J = 8.0, 1.2 Hz, 1H), 2.2 (s, 3H).
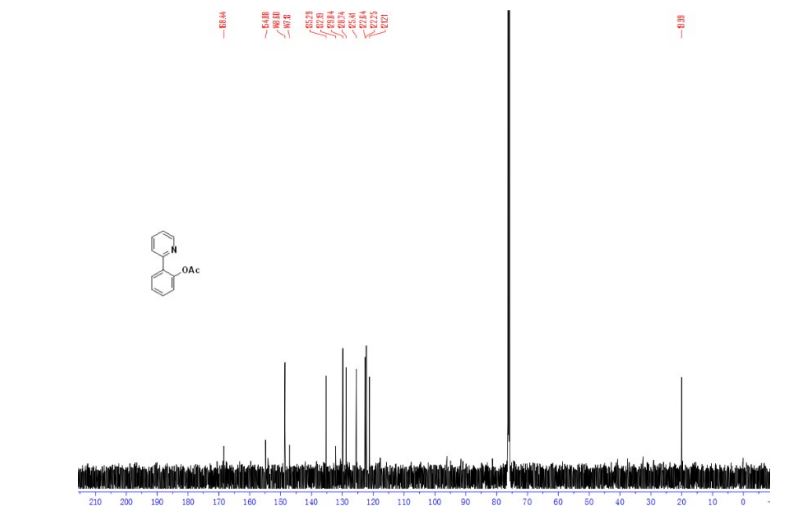
13C NMR (126 MHz, Chloroform-d) δ 168.4, 154.9, 148.6, 147.1, 135.3, 132.2, 129.8, 128.7, 125.4, 122.6, 122.3, 121.2, 20.0. GC-MS (EI) m/z: 213
//////////
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
 READ
READ
ANTHONY MELVIN CRASTO
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
 amcrasto@gmail.com
amcrasto@gmail.com
CALL +919323115463 INDIA
//////////////
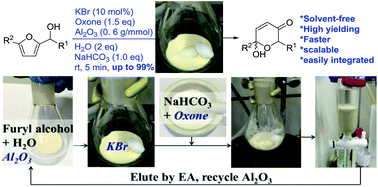
A solvent-free catalytic protocol for the Achmatowicz rearrangement
Abstract
Reported here is the development of an environmentally friendly catalytic (KBr/oxone) and solvent-free protocol for the Achmatowicz rearrangement (AchR). Different from all previous methods is that the use of chromatographic alumina (Al2O3) allows AchR to proceed smoothly in the absence of any organic solvent and therefore considerably facilitates the subsequent workup and purification with minimal environmental impacts. Importantly, this protocol allows for scaling up (from milligram to gram), recycling of the Al2O3, and integrating with other reactions in a one-pot sequential manner.
A solvent-free catalytic protocol for the
Achmatowicz rearrangement
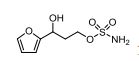
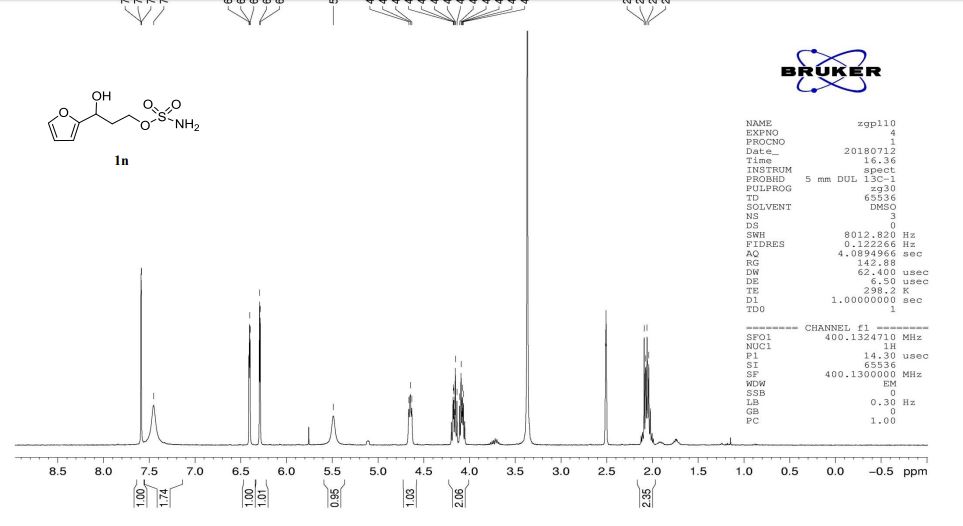
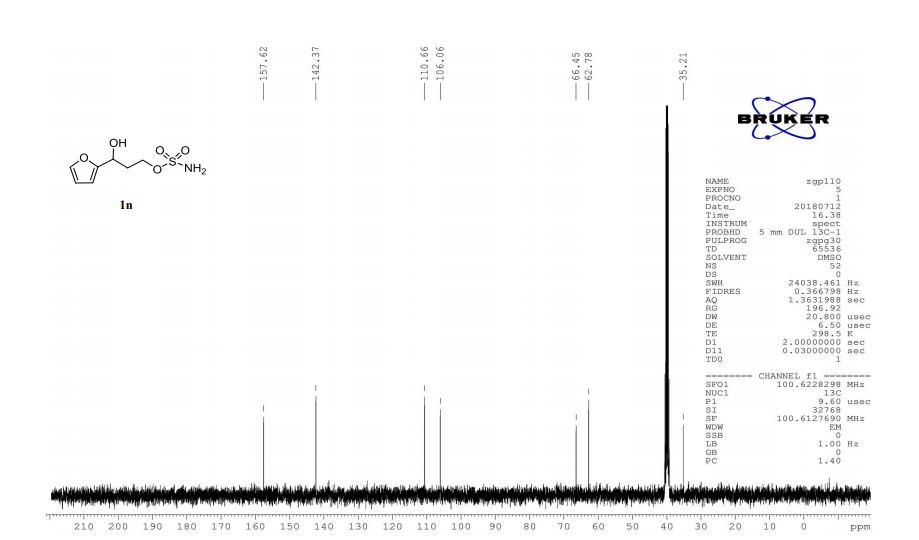
1n: colorless oil, 0.33 g, 73% yield for 2 steps.
1H-NMR (400 MHz, DMSO) δ: 7.59–7.58 (m, 1H), 7.45 (s, 2H), 6.40 (dd, J = 3.2, 1.8 Hz, 1H), 6.29 (d, J = 3.2 Hz, 1H), 5.49 (s, 1H), 4.74–4.60 (m, 1H), 4.18–4.07 (m, 2H), 2.09–2.04 (m, 2H).
13C-NMR (100 MHz, DMSO) δ: 157.6, 142.4, 110.7, 106.1, 66.5, 62.8, 35.2. IR (KBr) 3282.9, 2928.7, 1627.4, 1562.5, 1353.8, 1174.6, 1074.0, 999.7, 918.4, 742.8 cm-1 ;
HRMS (CI+ ) (m/z) calcd. for C7H11NO5S [M]+ 221.0352; found 221.0354.
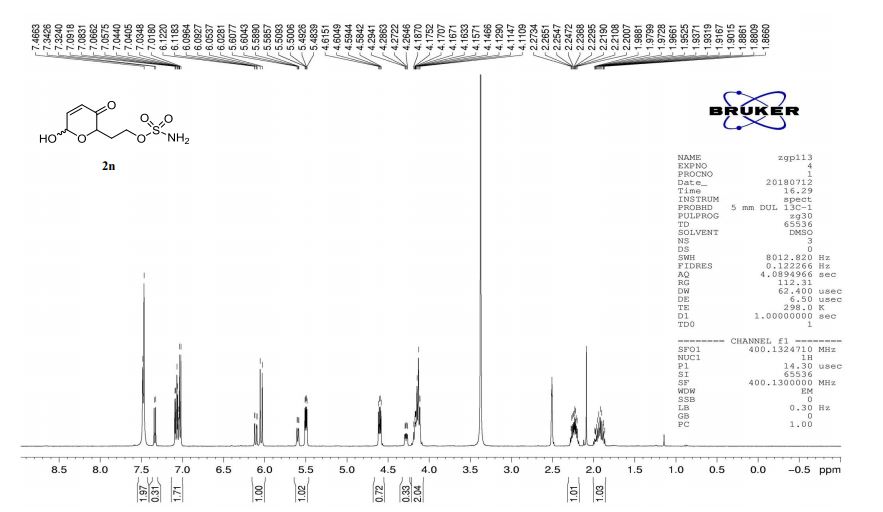
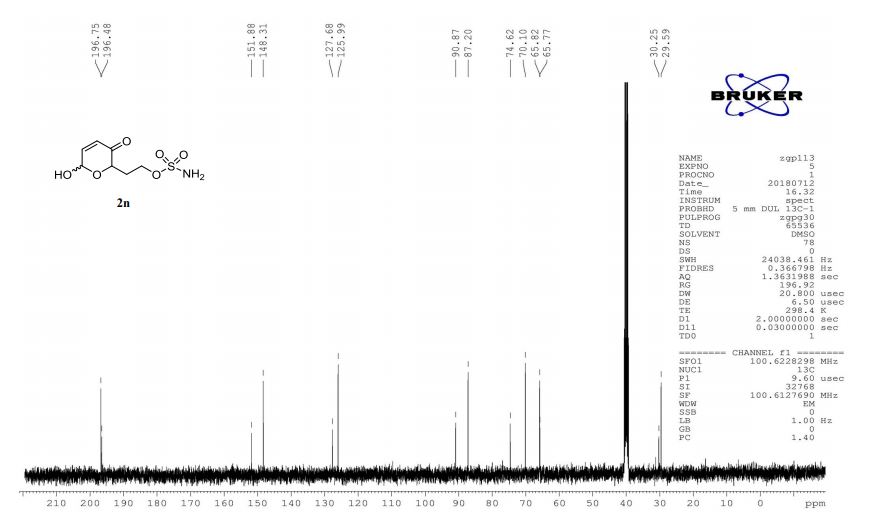
2n (EtOAc/hexane = 3:1):colorless oil (dr 7:3), 46 mg, 97%.
1H-NMR (400 MHz, DMSO) δ: 7.48–7.47 (m, 2H), 7.34–7.02 (m, 2H), 6.12–6.03 (m, 1H), 5.61–5.48 (m, 1H), 4.60 (dd, J = 8.3, 4.1 Hz, 0.7H), 4.28 (ddd, J = 8.8, 4.0, 1.3 Hz, 0.3H), 4.20–4.11 (m, 2H), 2.27–2.20 (m, 1H), 1.97–1.86 (m, 1H).
13C-NMR (100 MHz, DMSO) δ: 196.7, 196.5, 151.9, 148.3, 127.7, 126.0, 90.9, 87.2, 74.6, 70.1, 65.8, 65.8, 30.3, 29.6. IR (KBr) 3370.4, 2987.0, 1689.5, 1364.3, 1268.0, 1178.4, 1023.3, 928.3, 755.1 cm-1 ;
HRMS (CI+ ) (m/z) calcd. for C7H11NO6S [M]+ 237.0302; found 237.0315.
////////////////Achmatowicz rearrangement
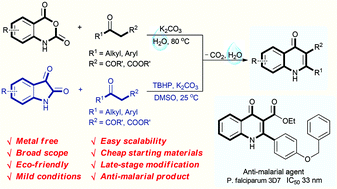
Eco-friendly decarboxylative cyclization in water: practical access to the anti-malarial 4-quinolones
Abstract
An environmentally benign decarboxylative cyclization in water has been developed to synthesize 4-quinolones from readily available isatoic anhydrides and 1,3-dicarbonyl compounds. Isatins are also compatible for the reaction to generate 4-quinolones in the presence of TBHP in DMSO. This protocol provides excellent yields under mild conditions for a broad scope of 4-quinolones, and has good functional group tolerance. Only un-harmful carbon dioxide and water are released in this procedure. Moreover, the newly synthesized products have also been selected for anti-malarial examination against the chloroquine drug-sensitive Plasmodium falciparum 3D7 strain. 3u is found to display excellent anti-malarial activity with an IC50 value of 33 nM.
Eco-friendly decarboxylative cyclization in water: practical access to the anti-malarial 4-quinolones
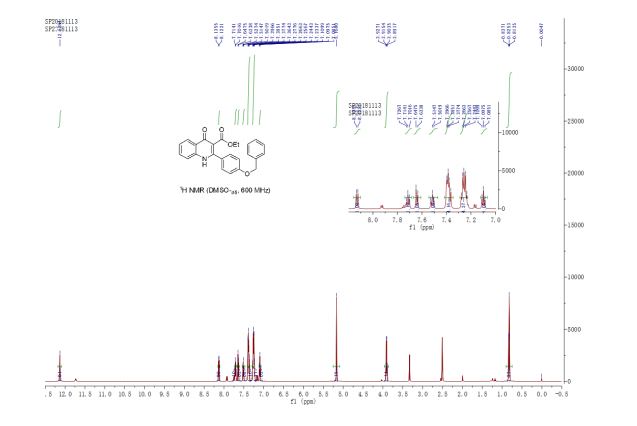
ethyl 2-(4-(benzyloxy)phenyl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (3u) White solid, m.p. 288-289 oC;
1H NMR (600 MHz, DMSO-d6) δ 12.14 (s, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.72 (ddd, J = 8.4, 7.1, 1.5 Hz, 1H), 7.64 (d, J = 8.3 Hz, 1H), 7.52 (td, J = 8.5, 1.7 Hz, 1H), 7.43 – 7.35 (m, 4H), 7.29 – 7.21 (m, 4H), 7.10 (td, J = 7.5, 0.5 Hz, 1H), 5.17 (s, 2H), 3.91 (q, J = 7.1 Hz, 2H), 2.00 (s, 1H), 0.83 (t, J = 7.1 Hz, 3H) ppm;
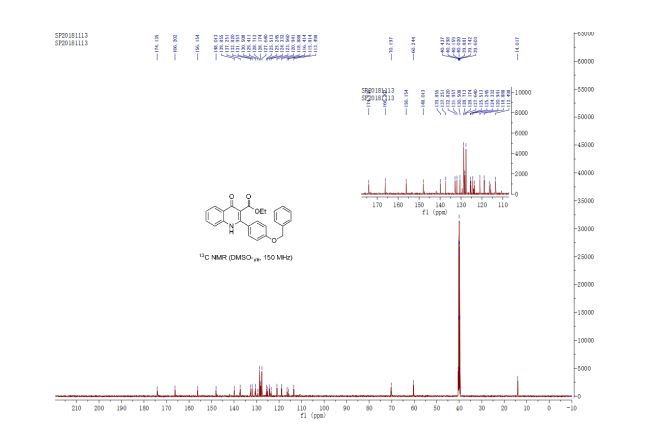
13C NMR (150 MHz, DMSO-d6) δ 174.1, 166.2, 156.2, 148.0, 139.8, 137.2, 132.8, 132.0, 130.5, 129.4, 128.7, 128.2, 127.6, 125.5, 125.2, 124.3, 123.6, 120.9, 118.9, 116.4, 115.8, 113.5, 70.2, 60.2, 14.0 ppm;
HRMS (ESI) calcd for [C25H21NO4+H]+ 400.1471, found 400.1463.
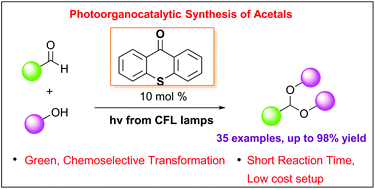
Photo-organocatalytic synthesis of acetals from aldehydes
Abstract
A mild and green photo-organocatalytic protocol for the highly efficient acetalization of aldehydes has been developed. Utilizing thioxanthenone as the photocatalyst and inexpensive household lamps as the light source, a variety of aromatic and aliphatic aldehydes have been converted into acyclic and cyclic acetals in high yields. The reaction mechanism was extensively studied
Photo-organocatalytic synthesis of acetals from aldehydes
(3,3-Dimethoxypropyl)benzene (2a)6
Colorless oil; 95% yield; 1H NMR (200 MHz, CDCl3) δ: 7.33-7.18 (5H, m, ArH), 4.37 (1H, t, J = 5.8 Hz, OCH), 3.33 (6H, s, 2 x OCH3), 2.68 (2H, t, J = 7.6 Hz, CH2), 1.98- 1.87 (2H, m, CH2); 13C NMR (50 MHz, CDCl3) δ: 141.8, 128.4, 125.9, 103.7, 52.8, 34.0, 30.8; MS (ESI) m/z 181 [M+H]+ .
6. Q. Zhou, T. Jia. X.-X. Li, L. Zhou, C.-J. Li, Y. S. Feng, Synth. Commun., 2018, 48, 1068.
.////////////////
Large scale synthesis of chiral (3Z,5Z)-2,7-dihydro-1H-azepine-derived Hamari ligand for general asymmetric synthesis of tailor-made amino acids.
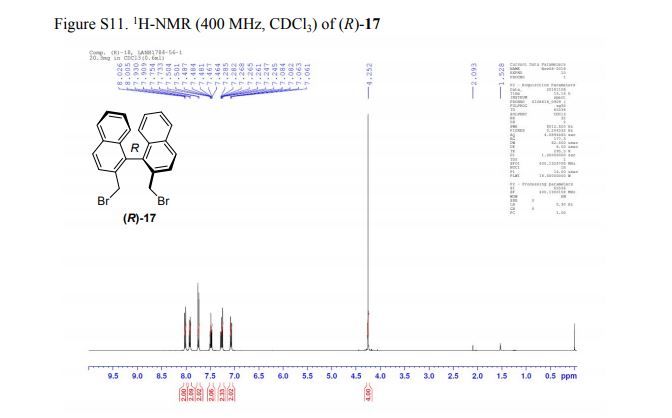
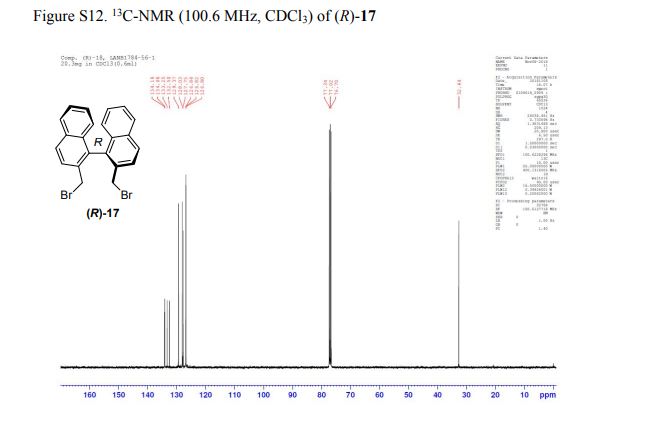
(R)-2,2′-bis(bromomethyl)-1,1′-binaphthalene ((R)-17) was prepared in the identical manner and had identical analytical properties to those given here.
1H NMR (400 MHz, CDCl3): δ 4.25 (4H, s, 2 × CH2), 7.07 (2H, dd, J = 8.4, 0.8 Hz, ArH), 7.27 (2H, ddd, J = 8.4, 6.8, 1.2 Hz, ArH), 7.48 (2H, ddd, J = 8.2, 6.8, 1.2 Hz, ArH), 7.74 (2H, d, J = 8.6 Hz, ArH), 7.92 (2H, d, J = 8.2 Hz, ArH), 8.02 (2H, d, J = 8.6 Hz, ArH).
13C NMR (100.6 MHz, CDCl3): δ 32.6 (CH2), 126.80 (ArCH), 126.82 (ArCH), 126.84 (ArCH), 127.7 (ArCH), 128.0 (ArCH), 129.4 (ArCH), 132.5 (quaternary ArC), 133.3 (quaternary ArC), 134.1 (quaternary ArC), 134.2 (quaternary ArC).
[α]20D = +173.8° (c = 1.0, CHCl3).
An advanced process for large scale (500 g) preparation of a (3Z,5Z)-2,7-dihydro-1H-azepine-derived chiral tridentate ligand (Hamari ligand), widely used for asymmetric synthesis of tailor-made α-amino acids via the corresponding glycine Schiff base Ni(II) complex, is disclosed. The process includes amidation, bis-alkylation, and precipitation/purification of the target compound by TFA as a counterion.
Large Scale Synthesis of Chiral (3Z,5Z)-2,7-Dihydro-1H-azepine-Derived Hamari Ligand for General Asymmetric Synthesis of Tailor-Made Amino Acids
//////////////////
(1S,4R)-2-(4-Methoxybenzoyl)bicyclo[2.2.2]octa-2,5-diene
(1S,4R)-2-(4-Methoxybenzoyl)bicyclo[2.2.2]octa-2,5-diene (3a) Yellow liquid (25.2 mg, 95% yield):
1H NMR (300 MHz, CDCl3) 1.39 (s, 4H, alkyl), 3.77-3.80 (m, 1H, alkyl), 3.85 (s, 3H, OMe), 4.36 (d, J = 5.4 Hz, 1H, alkyl), 6.36 (dd, J = 6.0, 6.0 Hz, 1H, vinyl), 6.46 (dd, J = 6.0, 6.0 Hz, 1H, vinyl), 6.88-6.91 (m, 3H, vinyl + arom.), 7.67 (d, J = 8.3 Hz, 2H, arom.);
13C{1H} NMR (75 MHz, CDCl3) = 24.7, 24.8, 37.1, 38.2, 55.4, 113.3, 130.8, 131.5, 133.2, 135.1, 146.5, 147.7, 162.6, 192.3;
HRMS (ESI-TOF) m/z calculated for C16H16NaO2 [M+Na]+ 263.1048, found 263.1036;
FT-IR (neat, cm-1 ) 1033, 1174, 1255, 1354, 1600, 1637, 1730, 2870, 2957, 3054.
Optical Rotation: []D 26 +39.9 (c 2.52, CHCl3) for an enantiomerically enriched sample of 94% ee.
HPLC analysis (column, CHIRALPAK AD-3, hexane/2-propanol = 98/2, flow rate 1.0 mL/min, 20 C, detection UV 250 nm light); tR of major-isomer 20.7
////////////////
(1S,4R)-2-(4-Methoxybenzoyl)bicyclo[2.2.2]octa-2,5-diene
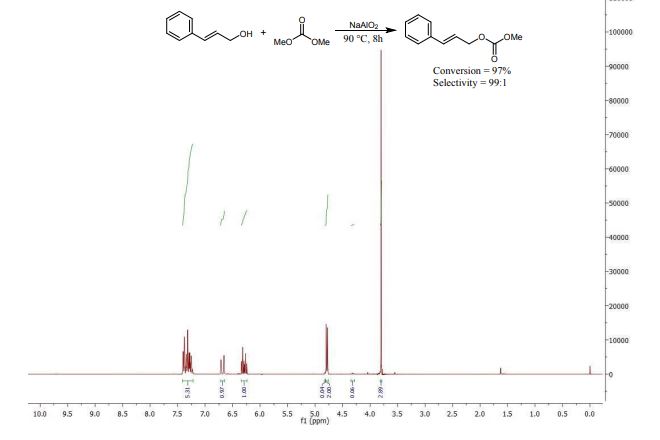
Sodium aluminate is presented as a highly active heterogeneous catalyst that is able to convert a range of alcohols into the corresponding unsymmetrical carbonate esters by reaction with dimethyl carbonate. Preparing NaAlO2 via spray drying boosts the basic properties and the activity of the catalyst.
https://pubs.acs.org/doi/10.1021/acs.oprd.8b00333
/////////////https://pubs.acs.org/doi/suppl/10.1021/acs.oprd.8b00333/suppl_file/op8b00333_si_001.pdf
carboxymethylation, dimethyl carbonate, mixed carbonate esters, sodium aluminate,
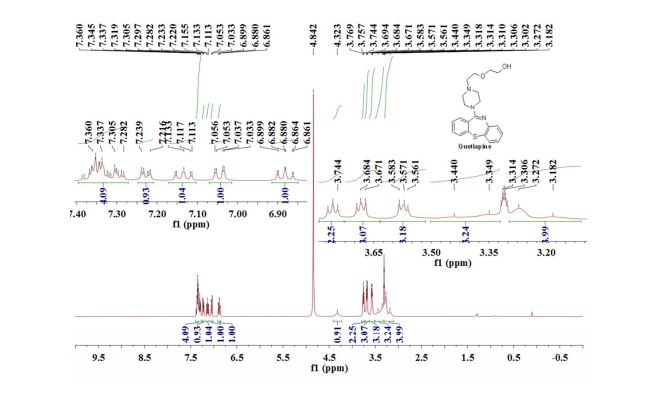
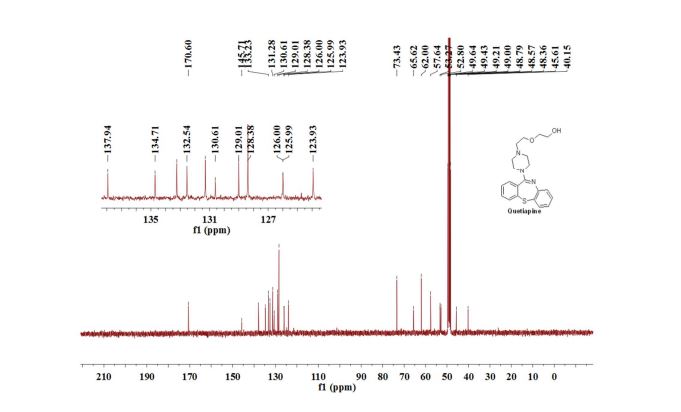
Quetiapine

Quetiapine
1H NMR (400 MHz, CD3OD): δ = 3.18-3.27 (m, 4H), 3.35-3.44 (m, 3H), 3.56-3.58 (m, 3H), 3.67-3.69 (m, 3H), 3.76 (t, J = 5.2 Hz, 2H), 4.32 (s, 1H), 6.88 (td, J = 7.4 Hz, 1.2 Hz, 1H), 7.04 (dd, J = 7.8 Hz, 1.6 Hz, 1H), 7.13 (td, J = 7.8 Hz, 1.6 Hz, 1H), 7.23 (dd, J = 6.8 Hz, 2.4 Hz, 1H), 7.28-7.39 (m, 4H) ppm.
13C NMR (100 MHz, CD3OD): δ = 40.2, 45.6, 52.8, 53.3, 57.6, 62.0, 65.6, 73.4, 123.9, 125.99, 126.0, 128.4, 129.0, 130.6, 131.3, 132.5, 133.2, 134.7, 137.9, 145.7, 170.6 ppm.
HRMS (ESI+ ): calcd for C21H26N3O2S [M+H]+ 384.1740, found 384.1735.
/////////////