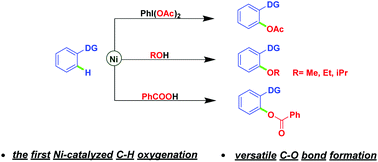
Nickel-catalyzed regioselective C–H oxygenation: new routes for versatile C–O bond formation
DOI: 10.1039/C8QO01274A, Research Article
Nickel-catalyzed regioselective C–H oxygenation reactions of chelating arenes using iodobenzene diacetate, alcohols, and benzoic acids respectively as attacking reagents have been developed for the first time.
To cite this article before page numbers are assigned, use the DOI form of citation above.
Abstract
Nickel-catalyzed regioselective C–H oxygenation reactions of chelating arenes using iodobenzene diacetate, alcohols, and benzoic acids respectively as attacking reagents have been developed for the first time. Simplicity of operation, broad range of functional group tolerance, use of cheap transition metal nickel, and avoiding extraneous directing groups are the key features, thus providing an important complement to C–H oxygenation reactions and expanding the field of nickel-catalyzed C–H functionalizations. Explorations of mechanistic details are also described.
Nickel-catalyzed regioselective C–H oxygenation: new routes for versatile C–O bond formation
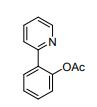
2-(pyridin-2-yl)phenyl acetate (2a)
Formula: C13H11NO2 Mass: 213
To a mixture of 2-phenylpyridine (77.5 mg, 0.5 mmol) 1a, Ni(acac)2 (25.7 mg, 0.1 mmol, 20 mol %), ligand MePh2P (20.0 mg, 0.1 mmol, 20 mol %), and PhI(OAc)2 (483.2 mg, 0.75 mmol, 1.5 equiv) in a reaction tube was added solvent (CH3CN=2.0 mL). The reaction mixture was stirred at 115 °C for 24 h in air. Following the general procedure, 2a was purified by column chromatography on silica gel (petroleum ether: ethyl acetate = 5:1) as a white solid (80.9 mg, 76%).
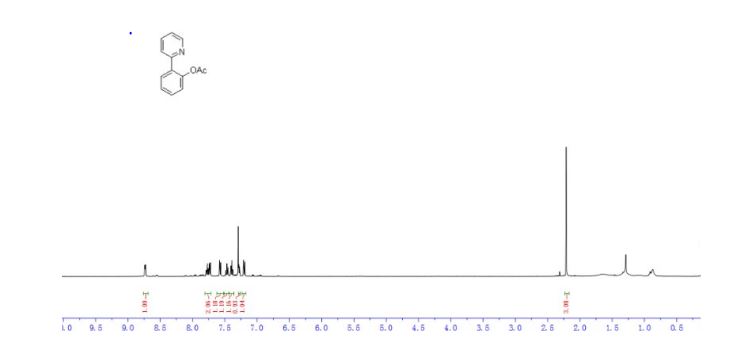
1H NMR (500 MHz, Chloroform-d) δ 8.8 – 8.7 (m, 1H), 7.8 – 7.7 (m, 2H), 7.6 (dd, J = 7.9, 1.1 Hz, 1H), 7.5 (td, J = 7.7, 1.7 Hz, 1H), 7.4 (td, J = 7.5, 1.2 Hz, 1H), 7.3 – 7.3 (m, 1H), 7.2 (dd, J = 8.0, 1.2 Hz, 1H), 2.2 (s, 3H).
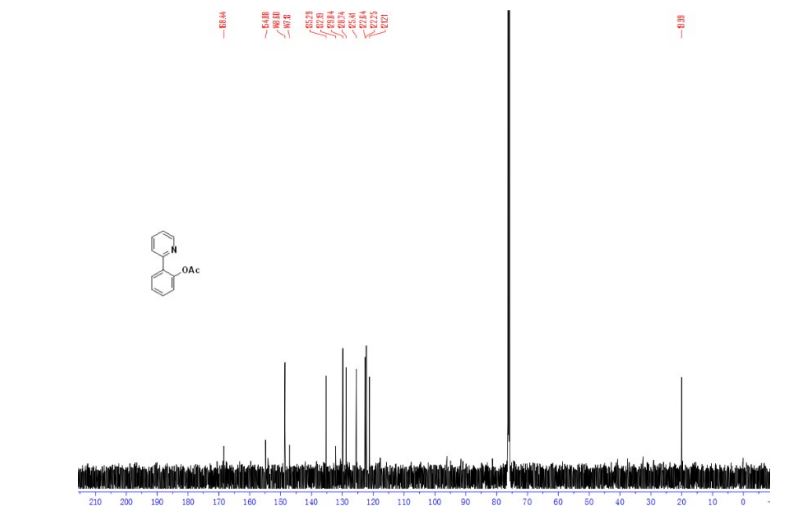
13C NMR (126 MHz, Chloroform-d) δ 168.4, 154.9, 148.6, 147.1, 135.3, 132.2, 129.8, 128.7, 125.4, 122.6, 122.3, 121.2, 20.0. GC-MS (EI) m/z: 213
//////////
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
 READ
READ
ANTHONY MELVIN CRASTO
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
 amcrasto@gmail.com
amcrasto@gmail.com
CALL +919323115463 INDIA
//////////////


















Sorry, the comment form is closed at this time.