WHAT IS PIC/S

PIC/S, Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme
History of PIC/S
PIC/S was founded in 1995 as an extension to PIC (Pharmaceutical Inspection Convention) which was founded in October 1970 by EFTA (European Free Trade Association) under the title of “The Convention for the Mutual Recognition of Inspections in Respect of the Manufacture of Pharmaceutical Products”.
What is PICS GMP?
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) is a non-binding, informal co-operative arrangement between Regulatory Authorities in the field of Good Manufacturing Practice (GMP) of medicinal products for human or veterinary use. It is open to any Authority having a comparable GMP inspection system.
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) was established in 1995 as an extension to the Pharmaceutical Inspection Convention (PIC) of 1970.
The Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (PIC/S) are two international instruments between countries and pharmaceutical inspection authorities. The PIC/S is meant as an instrument to improve co-operation in the field of Good Manufacturing Practices between regulatory authorities and the pharmaceutical industry.
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) is a non-binding, informal co-operative arrangement between Regulatory Authorities in the field of Good Manufacturing Practice (GMP) of medicinal products for human or veterinary use. It is open to any Authority having a comparable GMP inspection system. PIC/S presently comprises 54 Participating Authorities coming from all over the world (Europe, Africa, America, Asia and Australasia).
PIC/S aims at harmonising inspection procedures worldwide by developing common standards in the field of GMP and by providing training opportunities to Inspectors. It also aims at facilitating co-operation and networking between competent authorities, regional and international organisations, thus increasing mutual confidence. This is reflected in PIC/S’ mission which is to lead the international development, implementation and maintenance of harmonised GMP standards and quality systems of inspectorates in the field of medicinal products.
This is to be achieved by developing and promoting harmonised GMP standards and guidance documents; training competent authorities, in particular Inspectors; assessing (and reassessing) inspectorates and facilitating the co-operation and networking for competent authorities and international organisations.
The PIC/S’ mission is to lead the international development, implementation and maintenance of harmonised Good Manufacturing Practice (GMP) standards and quality systems of inspectorates in the field of medicinal products.
https://picscheme.org/docview/2146
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) was established in 1995 as an
extension to the Pharmaceutical Inspection Convention (PIC) of 1970.
PIC/S is a non-binding co-operative arrangement between Regulatory Authorities in the field
of Good Manufacturing Practice (GMP) of medicinal products for human or veterinary use.
It is open to any Authority having a comparable GMP inspection system.
PIC/S comprises more than 50 Participating Authorities coming from all over the world
(Europe, Africa, America, Asia and Australasia). The exact list of PIC/S Participating
Authorities is available on the PIC/S web site (www.picscheme.org).
PIC/S aims at harmonising inspection procedures worldwide by developing common
standards in the field of GMP and by providing training opportunities to inspectors.
It also aims at facilitating co-operation and networking between competent authorities,
regional and international organisations, thus increasing mutual confidence.
This is reflected in PIC/S’ mission which reads as follows: “To lead the international
development, implementation and maintenance of harmonised GMP standards and
quality systems of inspectorates in the field of medicinal products.”

As the Scheme is an arrangement between Regulatory Authorities, it is very flexible,
dynamic and proactive. A Committee of the Participating Authorities’
representatives (PIC/S Committee) supervises the operation of the Scheme.
All decisions are taken unanimously. The Committee is assisted in its task by 7 Sub-Committees
(e.g. on the training of inspectors, on GMDP harmonisation, etc.),
by an Executive Bureau, which steers the Organisation in-between meetings,
and by a small Secretariat, which mainly assists the Committee, the Sub-Committees, the
Bureau and Participating Authorities in their duties.
GMP is defined as follows in the PIC/S GMP Guide: “Good Manufacturing Practice is that
part of Quality Assurance which ensures that products are consistently produced and
controlled to the quality standards appropriate to their intended use and as required by the
marketing authorisation or product specification.”
Put in other words: GMP ensures that the production of medicines meets the required
quality standards.
The training of GMP inspectors has been at the heart of PIC/S since the beginning.
However, PIC/S has also opened some of its training tools to inspectors active in other
areas such as Good Distribution (GDP) and Good Clinical Practices (GCP).
All PIC/S training activities are regrouped under the PIC/S Inspectorates’ Academy (PIA) at
PIC/S arranges an annual Training Seminar for inspectors, with each Seminar dealing with
a specific topic. The Seminars are hosted by a different PIC/S Participating Authority each
year, as shown in the table below.
The annual PIC/S Seminar usually results in the setting-up of a Drafting Group, which
develops new or amends existing GMP guidance documents. For example, the 2004 PIC/S
Seminar on the Inspection of Active Pharmaceutical Ingredients (APIs) resulted in a PIC/S
guidance document for inspectors (Aide-Memoire on the Inspection of APIs).

Since its creation, PIC/S has been active in the development and promotion of harmonised
GMP standards and guidance documents.
In 1989, the EU adopted its own GMP Guide, which – in terms of GMP requirements – is
equivalent to the PIC/S GMP Guide. Since that time, the EU and the PIC/S GMP Guides
have been developed in parallel (both Guides are practically identical).
In addition to the GMP Guide, PIC/S has also been a pioneer in developing a number of
guidelines and guidance documents such as the Site Master File, the Recommendation
on Quality System Requirements for Pharmaceutical Inspectorates and the first
Guideline for the Manufacture of Active Pharmaceutical Ingredients. As a matter of fact,
PIC/S has been instrumental in elaborating a first draft for the ICH Q7A Guide on APIs,
which was finalised by ICH in 2000 and then adopted by PIC/S.

The main instrument for harmonisation has been the PIC/S GMP Guide. Originally, the latter
derives from the WHO GMP Guide and has been further developed in order to comply with
stringent manufacturing and health requirements, to cover new areas (e.g. biologicals) and to
adapt to scientific and industrial technology (e.g. biotech).
Other guidance documents are developed by ad-hoc Working Groups.
Current Working Groups are listed on:
www.picscheme.org/en/activities
The sharing of information between PIC/S Participating Authorities has become increasingly
important at a time when resources – whether in terms of staff or finance – are scarce.
The Scheme relies on the exchange of information on GMP inspections on a purely voluntary
basis. There is no obligation whatsoever to accept inspection results. There are also
important limitations to the exchange of information under PIC/S, notably the fact that it does
not apply to the exchange of information between the Participating Authorities of countries
party to the European Economic Area (EEA) and their MRA Partners. For these Authorities,
the EU legislation or the MRA is applicable – not PIC/S rules. However, the exchange of
information within PICS should become increasingly important to its Participating Authorities,
notably in connection with foreign inspections. A guidance on GMP inspection reliance was
adopted in 2018 with an aim to maximise inspection resources for GMP compliance
of overseas facilities.
The sharing of information between PIC/S Participating Authorities also applies to quality
defects of batches of medicinal products, which have been distributed on the market. Through
the PIC/S Rapid Alert and Recall System, such critical information is circulated among PIC/S
Participating Authorities, which are in a better position to oversee the withdrawal of the
defective batches from their markets.
HOW TO JOIN
Before an Authority is accepted by PIC/S, a detailed assessment is undertaken to determine
whether the Authority is able to apply an inspection system comparable to that of current
PIC/S Authorities. This assessment involves an examination of the Authority’s GMP
inspection and licensing system (or equivalent), quality system, legislative requirements,
inspector training, etc. It is followed by a visit by a PIC/S delegation to observe in particular
inspectors carrying out routine GMP inspections.
Membership may take several years to achieve, during which time various changes and
improvements may be recommended by the PIC/S Committee; if necessary, follow-up visits
are undertaken to verify the suitability of corrective actions.
PIC/S introduced in 2014 a pre-accession procedure to better prepare potentially interested
Authorities for PIC/S accession. The main advantage of the pre-accession procedure consists
in the carrying out of a pre-assessment by an auditor which includes a gap analysis allowing
to identify areas of non-compliance with PIC/S requirements.
In line with the Joint Reassessment Programme, existing PIC/S Participating Authorities
are also reassessed for equivalence on a regular basis. This ensures that both new applicants
and older members fulfil the same requirements
Pharmaceutical Inspection Co-operation Scheme (PIC/S)
14, rue du Roveray – 1207 GENEVA – Switzerland
Phone: +41 22 738 92 16
e-mail: info@picscheme.org
web site: www.picscheme.org
Mission
PIC/S’ mission is to be achieved by:
- Developing and promoting harmonised GMP standards and guidance documents (see “GMDP Harmonisation” and “Publications“);
- Training Competent Authorities, in particular GMP Inspectors (see “Training“);
- Assessing (and re-assessing) GMP Inspectorates (see “Accession” and “Compliance“);
- Facilitating co-operation and networking for Competent Authorities and International Organisations (see “International Co-operation“).
Vision and Values
PIC/S’ successful development has been possible due to the common sharing of the following shared vision and principles:
• A technical expert’s organisation: PIC/S has always taken great pride in featuring itself as a purely technical organisation in the field of regulatory GMP. Not becoming politically involved or discriminating against e.g. religion or race has always been PIC/S’ firm belief. Moreover, PIC/S is a major “think-tank” in the GMP field, the place where new ideas related to GMP are debated by highly competent experts.
• Based on consensus and mutual trust: Consensus in PIC/S has been based on the understanding that all Members have equal rights and obligations and that no Member is more “equal” (larger, richer…) than others. Despite the difficulty of having to negotiate compromises acceptable to all Members, PIC/S has always found a way forward without isolating a Member, which finds itself alone against the vast majority. Mutual trust is a key value in PIC/S and largely relies on the concept of:
a) voluntary co-operation (there is no legal obligation under PIC/S) and
b) each Member being assessed for equivalence before being admitted.
As all PIC/S Members are supposed to be equivalent, Members find it easier to exchange information on GMP on a voluntary basis.
• Driven by Members: PIC/S is an organisation which is mainly driven by Participating Regulatory Authorities and where the Secretariat has remained flexible and productive. As a result, PIC/S is a flexible and dynamic organisation, which is neither bureaucratic nor expensive. This also implies that Participating Authorities are expected to contribute to either PIC/S events (e.g. by hosting training events) or to PIC/S functioning (e.g. by allowing Members to carry out official duties for PIC/S such as chairing PIC/S meetings or representing PIC/S during conferences).
• Cemented by strong professional and personal links: PIC/S’ strength relies on its informal character, networking and the strong personal links between individual Members or Inspectors which have created a forum for brain storming, discussing new ideas and sharing information. It is not a coincidence that the first draft of the ICH Q7A Guide was initiated by PIC/S.
The initial Members of PIC comprised the 10 Member countries of EFTA at that time, ie. Austria, Denmark, Finland, Iceland, Liechtenstein, Norway, Portugal, Sweden, Switzerland and United Kingdom. Membership of PIC was subsequently expanded to include Hungary, Ireland, Romania, Germany, Italy, Belgium, France and Australia.
It was realised in the early 1990s that because of an incompatibility between the Convention and European law, it was not possible for new countries to be admitted as Members of PIC. Australia was the last country that was able to become a Member of PIC in January 1993. Consequently, the PIC Scheme was formed on 2 November 1995. PIC and the PIC Scheme, which operate together in parallel, are jointly referred to as PIC/S.

The original goals of PIC were:
- Mutual recognition of inspections;
- Harmonisation of GMP requirements;
- Uniform inspection systems;
- Training of Inspectors;
- Exchange of information;
- Mutual confidence.
These goals have also been adopted by the PIC Scheme and expanded to include additional goals. The PIC Convention, dated October 1970, is available below.
Information on the dates of accession of PIC/S Participating Authorities are available under “Members“.
Benefits
PIC/S offers a variety of advantages to its Participating Authorities. Some of the main benefits for Medicines Regulatory Authorities resulting from PIC/S Membership are detailed below. PIC/S Membership also involves indirect benefits to industry, when their relevant Medicines Regulatory Authority becomes a Member of PIC/S.
Main benefits for Members
- Training opportunities: PIC/S provides a forum for the training of GMP Inspectors thus allowing the latter to benefit from increased training opportunities by attending PIC/S Seminars and Expert Circles and by participating in the PIC/S Joint Visits Programme. In this respect, PIC/S is unique as there is no other international training forum run jointly by Regulatory Authorities (individually, Regulatory Authorities or organisations such as WHO or the EMA provide basic training courses, mainly to new Inspectors).
- International GMP harmonisation: By taking part in the meetings of the PIC/S Committee, PIC/S Participating Authorities are involved in the development and harmonisation of international GMP guides and guidelines. The PIC/S Committee also actively promotes the uniform interpretation of GMP and Quality Systems for GMP Inspectorates.
- Networking: By attending PIC/S activities, participants benefit from personal contacts with other agencies, whether they are part of PIC/S or not. This networking often simplifies contacts and the exchange of GMP related information. In addition, PIC/S is one of the few international GMP fora for networking and confidence building amongst Regulatory Inspectors where experts (GMP Inspectors, specialist GMP Inspectors and Chief Inspectors) can meet, discuss issues of mutual concern and share experiences and information. In other fora, participation is either at the level of Heads of Agencies (e.g. WHO) or at the level of experts in a particular field (ICH).
- High standards: PIC/S ensures that all Members comply with PIC/S standards at all times (assessment of new applicants and reassessment of existing Member Inspectorates). Preparing for the accession to the Scheme (or reassessment) forces improvements in the GMP inspection system and procedures. This results in increased efficiency of the GMP Inspectorate. This is particularly true for Quality System requirements, where PIC/S standards are high, and for GMP training, which is essential in PIC/S.
- Sharing of information: PIC/S allows for a more effective use of inspection resources through the voluntary sharing of GMP inspections reports. Membership is also a cost-saving measure for the inspection authorities confronted with an increase of inspections, notably in the field of Active Pharmaceutical Ingredients (APIs).
- Rapid Alert System: Through PIC/S Membership, Regulatory Authorities automatically benefit from being part of the PIC/S Rapid Alert and Recall System arising from quality defects of batches of medicinal products, which have been distributed on the market. The PIC/S Alert and Recall System is part of a wider system, which includes the Alert and Recall System of EU/EEA/MRA partners.
- Facilitating the conclusion of other Agreements: Membership in PIC/S may also facilitate the conclusion of other agreements, e.g. Mutual Recognition Agreements, between Members at various levels (e.g. Australia-Canada MRA, EU-Switzerland MRA, etc.). During the recently concluded initial negotiation on ASEAN MRA on GMP Inspection, PIC/S Membership accession was accepted as one of the essential criteria for MRA.
Indirect Benefits for Industry
There are also indirect benefits to industry when their relevant Regulatory Authority becomes a Member of PIC/S. These benefits may include the following:
- Reduced duplication of inspections;
- Cost savings;
- Export facilitation;
- Enhanced market access.
Although PIC/S is not a trade agreement, Membership in PIC/S may facilitate the export of pharmaceuticals. Some non-PIC/S Authorities accept GMP Certificates from PIC/S Participating Authorities. This means that non-PIC/S Authorities and organisations have a greater confidence in medicines manufactured in countries where the Regulatory Authority is a PIC/S Participating Authority. Consequently, the pharmaceutical industry located in these countries indirectly benefits from PIC/S Membership.
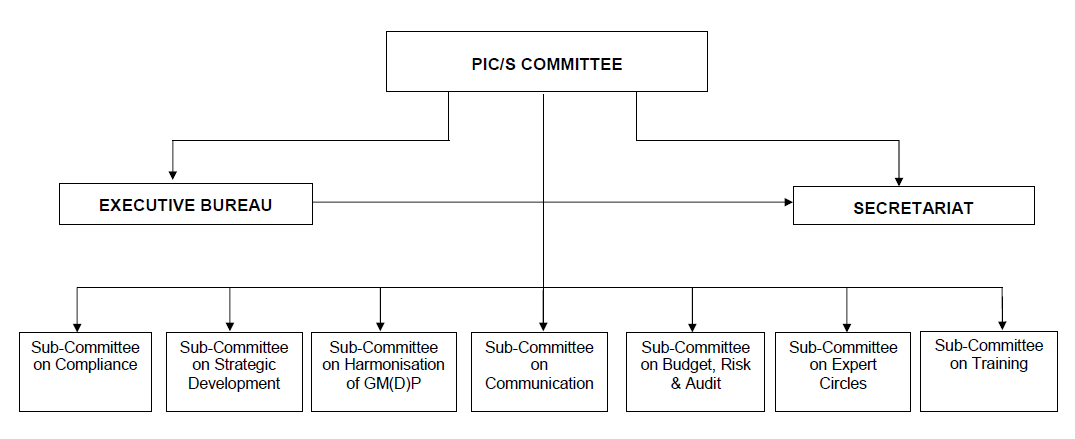
Organisational Structure
As the PIC Scheme is an arrangement between Regulatory Authorities, it is very flexible, dynamic and proactive. A Committee of the Participating Authorities’ representatives (PIC/S Committee) supervises the operation of the Scheme. All decisions are taken unanimously. The Committee is assisted in its task by 7 Sub-Committees (e.g. on the training of Inspectors, on GMDP harmonisation, etc.), by an Executive Bureau, which steers the Organisation in between meetings, and by a small Secretariat, which mainly assists the Committee, the Sub-Committees, the Bureau and Participating Authorities in their duties.
International Co-operation
PIC/S has been a pioneer organisation in the field of pharmaceutical inspections and Good Manufacturing Practice (GMP). It has successfully adapted to a constantly changing environment, in particular globalisation. It is the only organisation worldwide which exclusively deals with GMP. This is also reflected in PIC/S’ mandate which is “to lead the international development, implementation and maintenance of harmonised Good Manufacturing Practice (GMP) standards and quality systems of Inspectorates in the field of medicinal products.”
As mentioned in the PIC/S Blueprint (see “Publications“), the increasing globalisation of public health concerns and the multiplication of actors involved (both in industry and among Regulatory Authorities) makes it necessary to further increase harmonisation efforts in setting regulatory requirements, inspecting and evaluating GMP compliance, licensing manufacturing sites, recalling defective batches and increasing the exchange of information. PIC/S offers an attractive platform to respond to the challenges of globalisation.
PIC/S is, however, not alone to handle GMP. There are other organisations, which are also involved in this field, although – unlike PIC/S – the GMP part of their activities may only represent a fraction of their total activities.
PIC/S has relations with the following organisations (in alphabetical order):
| ASEAN | Association of South East Asian Nations |
| EC | European Commission |
| ECA | European Compliance Academy |
| EDQM | European Directorate for the Quality of Medicines |
| EMA | European Medicines Agency |
| EU HMA | Heads of Medicines Agencies of the European Union |
| ICH | International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use |
| ICMRA | International Coalition of Medicines Regulatory Authorities |
| IFPMA | International Federation of Pharmaceutical Manufacturers Associations |
| ISPE | International Society for Pharmaceutical Engineering |
| PDA | Parenteral Drug Association |
| OIE | World Organisation for Animal Health |
| UNICEF | United Nations International Children’s Emergency Fund |
| WHO | World Health Organization |
While relations with the European Union are of a privileged nature, due to the fact that many PIC/S Participating Authorities are also Competent Authorities of EU Member States, PIC/S aims at remaining impartial and avoiding to give more importance to one organisation over another. All organisations are treated equally.
Co-operation between PIC/S and other organisations are based on the principle of complementarity. Where PIC/S Participating Authorities are already actively co-operating, PIC/S as an organisation strives to not duplicate Members’ efforts. As human and financial resources are getting scarce at the level of national authorities as well as at the level of international organisations, the duplication of tasks with other organisations must be avoided at all costs.
PIC/S is thus increasingly focusing on its added-value and specificity. At the same time, in a world, which has become increasingly globalised, PIC/S aims at turning into a more global and efficient organisation and harmonise international efforts in the field of GMP. This is what PIC/S aims to achieve through its international co-operation efforts.
Associated Partner Organisations
PIC/S has signed co-operation agreement with the following four organisations:
- European Medicines Agency (EMA);
- European Directorate for the Quality of Medicines (EDQM);
- World Organisation for Animal Health (OIE);
- United Nations International Children’s Emergency Fund (UNICEF);
- World Health Organization (WHO).
Other Organisations
Without having institutionalised its relations, PIC/S co-operates with an increasing number of organisations, which are also active in the field of GMDP…. More
Professional Organisations & Industry
Since the manufacturer is at the heart of the GMP process, it is essential for PIC/S to maintain good relations with industry and professional organisations, notably those more active in the field of regulation, guidance documents and training. The support of industry associations and professional organisations to the goals of PIC/S is a prerequisite for the latter’s successful expansion. As a matter of fact, industry is generally favourable to PIC/S’ expansion: the harmonisation of GMP facilitates trade and also means fewer inspections for manufacturers. The latter are increasingly worried by the multiplication and duplication of inspections, which means not only more fees but also more staff immobilised and possibly a lower-than-usual production output during these inspections…. More
History
The PIC (Pharmaceutical Inspection Convention) was founded in October 1970 by the European Free Trade Association (EFTA), under the title of the Convention for the Mutual Recognition of Inspections in Respect of the Manufacture of Pharmaceutical Products.[1][2] The initial members comprised the 10 member countries of EFTA at that time. In the early 1990s it was realized that because of an incompatibility between the Convention and European law, it was not possible for new countries to be admitted as members of PIC. European law did not permit individual EU countries that were members of PIC to sign agreements with other countries seeking to join PIC. As a consequence the Pharmaceutical Inspection Co-operation Scheme was formed on 2 November 1995. The Pharmaceutical Inspection Co-operation Scheme is an informal agreement between health authorities instead of a formal treaty between countries. PIC and the PIC Scheme, which operate together in parallel, are jointly referred to as PIC/S. PIC/S became operational in November 1995.[2]
Since its conception until 2003, PIC/S did not have a distinct legal identity. Its Secretariat was provided by the European Free Trade Association. Based on PIC/S meeting in June 2003, its committee decided to constitute itself as a Swiss Association in accordance with article 60 of the Swiss Civil Code which refer to other internationally active organizations established in Switzerland such as the International Committee of the Red Cross (ICRC). On 1 January 2004, PIC/S established its own Secretariat in Geneva, Switzerland.[3]
Purpose
PIC/S has a number of provisions intended to establish the following:[4]
- Mutual recognition of inspection between member countries, so that an inspection carried out by officials of one member country will be recognized as valid by other members.
- Equivalent principles of inspection methodology, so that it is understood that inspectors in each member country will be following the same best practices when carrying out inspections.
- Mechanisms for the training of inspectors.
- Harmonization of written standards of Good Manufacturing Practices.
- Lines of communication between member country inspectors/inspectorates.
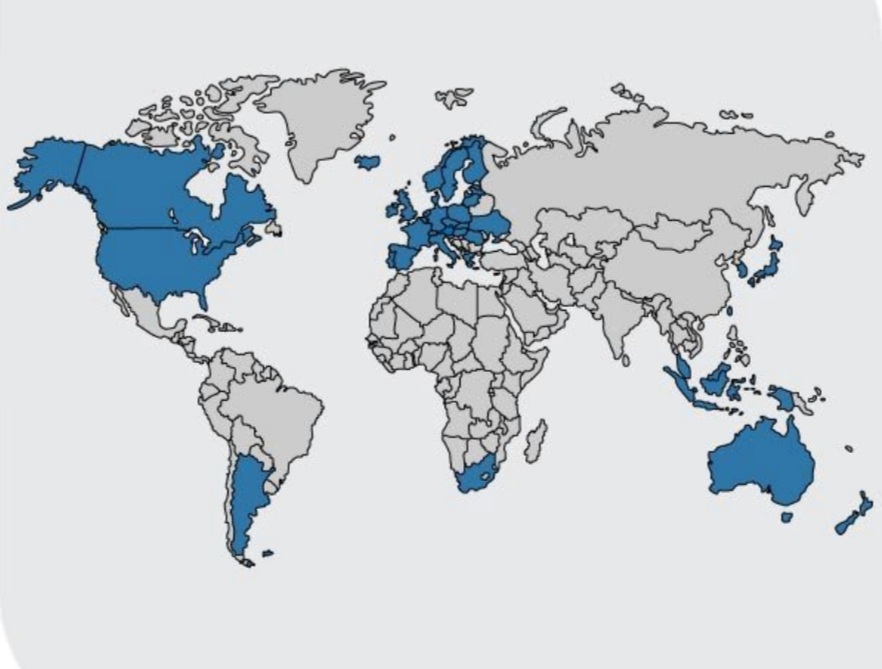
Members
Map of the members of PIC/S
The following are the state members of PIC/S as of October 2021:[5]
| Country | Participating entity | Accession to PIC Scheme |
|---|---|---|
| National Institute of Drugs Instituto Nacional de Medicamentos (INAME) |
2008 | |
| Therapeutic Goods Administration (TGA) | 1995 | |
| Federal Office for Safety in Health Care Bundesamt für Sicherheit im Gesundheitswesen (BASG) |
1999 | |
| Federal Agency for Medicines and Health Products Agence Fédérale des Médicaments et des Produits de Santé (AFMPS) Federaal Agentschap voor Geneesmiddelen en Gezondheidsproducten (FAGG) |
1997 | |
| National Health Surveillance Agency Agência Nacional de Vigilância Sanitária (ANVISA) |
2021 | |
| Health Canada‘s Regulatory Operations and Enforcement Branch (ROEB) Santé Canada Direction générale des opérations réglementaires et de l’application de la loi (DGORAL) |
1999 | |
| Food and Drug Administration (TFDA) | 2013 | |
| Agency for Medicinal Products and Medical Devices of Croatia Agencija za lijekove i medicinske proizvode (HALMED) |
2016 | |
| Pharmaceutical Services (CyPHS) | 2008 | |
| State Institute for Drug Control Státní Ústav pro Kontrolu Léčiv (SÚKL) Institute for State Control of Veterinary Biologicals and Medicines (ISCVBM) |
1997 2005 |
|
| Danish Medicines Agency (DKMA) | 1995 | |
| State Agency of Medicines (SAM) | 2007 | |
| Finnish Medicines Agency (FIMEA) | 1996 | |
| French National Agency for Medicines and Health Products Safety Agence nationale de sécurité du médicament et des produits de santé (ANSM) Agency for Food, Environmental & Occupational Health Safety Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail (ANSES) |
1997 2009 |
|
| Central Authority of the Laender for Health Protection regarding Medicinal Products and Medical Devices Zentralstelle der Länder für Gesundheitsschutz bei Arzneimitteln und Medizinprodukten (ZLG) |
2000 | |
| Greek National Organisation for Medicines Εθνικός Οργανισμός Φαρμάκων (EOF) |
2002 | |
| Pharmacy and Poisons Board of Hong Kong (PPBHK) | 2016 | |
| National Institute of Pharmacy and Nutrition (OGYÉI) | 1995 | |
| Icelandic Medicines Agency (IMA) | 1995 | |
| National Agency for Drug and Food Control (NADFC) Badan Pengawas Obat dan Makanan Republik Indonesia (BPOM) |
2012 | |
| Iran Food and Drug Administration (IFDA) | 2018 | |
| Health Products Regulatory Authority (HPRA) | 1996 | |
| Institute for Standardization and Control of Pharmaceuticals (ISCP) | 2009 | |
| Italian Medicines Agency Agenzia Italiana del Farmaco (AIFA) Directorate General for Animal Health and Veterinary Medicinal Products Direzione generale della sanità animale e dei farmaci veterinari (DGSAF) |
2000 2020 |
|
| Pharmaceuticals and Medical Devices Agency (PMDA) | 2014 | |
| State Agency of Medicines Zāļu valsts aģentūra (ZVA) |
2004 | |
| Office of Healthcare Amt für Gesundheit (AG) |
1995 | |
| State Medicines Control Agency (SMCA) | 2009 | |
| National Pharmaceutical Regulatory Agency (NPRA) Bahagian Regulatori Farmasi Negara |
2002 | |
| Malta Medicines Authority (MMA) | 2008 | |
| Federal Commission for the Protection against Sanitary Risk Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS) |
2018 | |
| Health and Youth Care Inspectorate Inspectie Gezondheidszorg en Jeugd (IGJ) |
1995 | |
| Medicines and Medical Devices Safety Authority (Medsafe) | 2013 | |
| Norwegian Medicines Agency (NOMA) | 1995 | |
| Chief Pharmaceutical Inspectorate (CPI) | 2006 | |
| National Authority of Medicines and Health Products, IP Autoridade Nacional do Medicamento e Produtos de Saúde IP (INFARMED IP ) |
1999 | |
| National Agency for Medicines and Medical Devices (NAMMD) | 1995 | |
| Health Sciences Authority (HSA) | 2000 | |
| State Institute for Drug Control (SIDC) | 1997 | |
| Agency for Medicinal Products and Medical Devices Javna agencija Republike Slovenije za zdravila in medicinske pripomočke (JAZMP) |
2012 | |
| South African Health Products Regulatory Authority (SAHPRA) | 2007 | |
| Ministry of Food and Drug Safety (MFDS) | 2014 | |
| Spanish Agency of Medicines and Medical Devices Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) |
1998 | |
| Swedish Medical Products Agency (MPA) | 1996 | |
| Swiss Agency for Therapeutic Products (Swissmedic) | 1996 | |
| Food and Drug Administration (Thai FDA) | 2016 | |
| Turkish Medicines and Medical Devices Agency (TMMDA) | 2018 | |
| State Service for Medications and Drugs Control (SMDC) | 2011 | |
| Medicines and Healthcare products Regulatory Agency (MHRA) Veterinary Medicines Directorate (VMD) |
1999 2014 |
|
| U.S. Food and Drug Administration (USFDA) | 2011 |
See also
- GxP
- Good automated manufacturing practice (GAMP)
- Corrective and preventive action (CAPA)
- Validation (drug manufacture)
- European Medicines Agency (EMEA)
- European Federation of Pharmaceutical Industries and Associations (EFPIA)
- Pharmaceutical Research and Manufacturers of America (PhRMA)
References
- ^ “History of PIC/S”. picscheme.org. Retrieved 2 October 2021.
- ^ Jump up to:a b Brunner, Daniel (September 2004). “Pharmaceutical Inspection Co-operation Scheme (PIC/S)”. The Quality Assurance Journal. 8 (3): 207–211.
- ^ “Legal Form”. picscheme.org. Retrieved 2 October 2021.
- ^ McCormick, Kate (2002). Quality (Pharmaceutical Engineering Series). Butterworth-Heinemann. p. 158. ISBN 9780750651134.
- ^ “Members”. www.picscheme.org. Retrieved 2 October 2021.
External links
- Official website
- European Federation of Pharmaceutical Industries and Associations (EFPIA)
- Japan Pharmaceutical Manufacturers Association (JPMA)
- Pharmaceutical Research and Manufacturers of America (PhRMA)
Links
| A |
| American Association of Pharmaceutical Sciences (AAPS) |
| Active Pharmaceutical Ingredients Committee (APIC) |
| C |
| CEFIC |
| F |
| Food and Drug Administration, USA (FDA) – CDER |
| FDA Guidance Documents |
| FDA Inspection Guides |
| FDA Warning Letters |
| I |
| International Conference on Harmonisation (ICH) |
| International Federation for Animal Health (IFAH) |
| International Society of Pharmaceutical Engineers (ISPE) |
List of Acronyms
| A | |
| API(s) | Active Pharmaceutical Ingredients |
| ASEAN | Association of South East Asian Nations |
| C | |
| CHF | Swiss Francs |
| D | |
| DIA | Drugs Information Association |
| E | |
| EC | European Commission |
| EEC | European Economic Community |
| EDQM | European Directorate for the Quality of Medicines |
| EEA | European Economic Area |
| EFTA | European Free Trade Association |
| EMA | European Medicines Agency |
| EU | European Union |
| F | |
| FDA | Food and Drug Administration |
| FIP | Fédération Internationale Pharmaceutique |
| G | |
| GCP | Good Clinical Practices |
| GCLP | Good Control of Laboratory Practice |
| GDP | Good Distribution Practice |
| GLP | Good Laboratory Practices |
| GMP | Good Manufacturing Practice |
| I | |
| ICH | International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use |
| IFPMA | International Federation of Pharmaceutical Manufacturers Associations |
| IMID | International Medicinal Inspectorates’ Database |
| ISPE | International Society for Pharmaceutical Engineering |
| IQ | Installation Qualification |
| J | |
| JVP | Joints Visits Programme |
| M | |
| MERCOSUR | Mercado Común del Sur |
| MoU | Memorandum of Understanding |
| MRA(s) | Mutual Recognition Agreement(s) |
| N | |
| NAFTA | North American Free Trade Agreement |
| O | |
| OECD | Organisation for Economic Co-operation and Development |
| OQ | Operational Qualification |
| P | |
| PA | Participating Authority |
| PAHO | Pan American Health Organization |
| PIC | Pharmaceutical Inspection Convention |
| PIC/S | Pharmaceutical Inspection Co-operation Scheme |
| PDA | Parenteral Drug Association |
| R | |
| RA | Regulatory Authority |
| Q | |
| QA | Quality Assurance |
| Q7A | ICH Expert Working Group on Active Pharmaceutical Ingredients |
| U | |
| UNICEF | United Nations International Children’s Emergency Fund |
| W | |
| WHO | World Health Organization |
| WTO | World Trade Organization |
| Logo of the Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme | |
| Abbreviation | PIC/S |
|---|---|
| Formation | 26 May 1971 (50 years ago) |
| Type | Intergovernmental organization |
| Purpose | Pharmaceutical |
| Headquarters | Geneva, Switzerland |
| Coordinates |  46.2050823°N 6.158363°ECoordinates: 46.2050823°N 6.158363°ECoordinates:  46.2050823°N 6.158363°E 46.2050823°N 6.158363°E |
| Area served | International |
| Membership | 52 Active state members |
| Website | www.picscheme.org |
https://picscheme.org/docview/3486
Contact
PIC/S Secretariat
14, rue du Roveray CH
1207 Geneva
Switzerland
Tel: (+41) 22 738 92 16
Email: info@picscheme.org
PIC/S, Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation
2,182 total views, 3 views today












