A roadmap towards green packaging: the current status and future outlook for polyesters in the packaging industry
Green Chem., 2017, 19,4737-4753
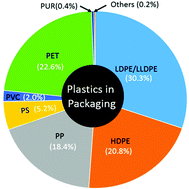
Approximately 99% of the plastics used in the packaging industry today are petroleum-based. However, the adoption of biobased plastics could help to greatly reduce the environmental footprint of packaging materials and help to conserve our non-renewable petroleum resources. This tutorial review provides an overview of renewable polyesters and their potential packaging materials.
A roadmap towards green packaging: the current status and future outlook for polyesters in the packaging industry
Muhammad Rabnawaz
Assistant Professor

rabnawaz@msu.edu
Telephone: 517-432-4870
Rabnawaz’s Research Group
School of Packaging

Dr. Cheng earned his PhD from South Dakota State University in May 2017. He has extensive research experiences in biomass pyrolysis and liquefaction, bio-oil catalytic cracking and hydrodeoxygenation, catalyst design, preparation, characterization and evaluation, food extruding, nano cellulose and protein peptides production, polymer synthesis, characterization and application.
Project Titles worked on: Innovation for Improved Sustainability: Scalable Approach for the Preparation of Thermoplastic Starches and their Composites for Applications in Biodegradable Packaging .
Duration in the group: August 2017- Present
Areas of Interest: Polycarbonates and polyesters synthesis, characterization and application.
MSU email Id: chengsho@msu.edu
 |
Ian Wyman
Education: Ph.D., Queen’s University, Kingston, Ontario Email: wymani@chem.queensu.ca |
Abstract
Approximately 99% of the plastics produced today are petroleum-based, and the packaging industry alone consumes over 38% of these plastics. In this review, we argue that renewable polyesters can provide a key milestone as renewable plastics in the route toward green packaging. This review describes different classes of polyesters with particular regard to their potential use as packaging materials. Some of the families of polyesters discussed include poly(ethylene terephthalate) and its renewable analogs, poly(lactic acid), poly(hydroxyalkanoates), and poly(epoxy anhydrides). The synthesis of polyesters is discussed from a green chemistry perspective. A structure–property correlation among the various polyesters is also discussed. The challenges that currently hinder the widespread adoption of polyesters as leading packaging materials are reviewed. The environmental footprint and end of life scenario of polyesters are discussed. Finally, future research directions are summarized as a possible roadmap towards the widespread adoption of renewable polyesters as sustainable packaging materials.
////////////
Muhammad Rabnawaz
Assistant Professor

rabnawaz@msu.edu
Telephone: 517-432-4870
Rabnawaz’s Research Group
School of Packaging
Research Interests
I have published more than 20 research articles in the field of polymer and materials sciences. Our initial endeavors can be divided into three broad categories:
- Polymer synthesis from renewable feedstocks.
- Design and preparation of smart materials.
- Polymer composites.
Our projects are highly applied, and we expect close collaboration with world-leading industries. These partnerships will offer unique training and career opportunities for the group members.
Experience
- Assistant Professor, School of Packaging, Michigan State University (2016-currrent)
- Postdoctorate, University of Illinois, Urbana-Champaign, 2015-2016
- Postdoctorate, Queen’s University, Canada, 2013-2015
Education
- Ph.D., Chemistry, Queen’s University, Canada, 2013
- M.Sc., Chemistry, University of Peshawar, Pakistan, 2004