Oramed Submits Pre-IND Package to FDA for ORMD-0901 (oral exenatide), an …
MarketWatch
JERUSALEM, September 3, 2013 /PRNewswire via COMTEX/ — Oramed Pharmaceuticals Inc. (nasdaqcm:ORMP) (http://www.oramed.com), a developer of oral drug delivery systems, announced today that it has submitted a pre-Investigational New Drug … capsule …
Experimental Drug Shows Promise for Rare Genetic Disorder

Transthyretin, or TTR for amyloidosis
THURSDAY Aug. 29, 2013 — A new medication appears to be highly effective in combating a heredity-based form of the organ-damaging genetic disorder known as amyloidosis, according to researchers.
Amyloidosis refers to a family of more than a dozen diseases in which different types of abnormal proteins called amyloids lodge in major organs and nerves. These amyloids build up to the point that they cause damage and, ultimately, organ failure.
read all at
http://www.drugs.com/news/experimental-shows-promise-rare-genetic-disorder-47059.html
Transthyretin (TTR) is a serum and cerebrospinal fluid carrier of the thyroid hormone thyroxine (T4) and retinol-binding protein bound to retinol. This is how transthyretin gained its name, transports thyroxine and retinol. The liver secretes transthyretin into the blood, and the choroid plexus secretes TTR into thecerebrospinal fluid.
TTR was originally called prealbumin[1] (or thyroxine-binding prealbumin) because it ran faster than albumin on electrophoresis gels.
Binding affinities
It functions in concert with two other thyroid hormone-binding proteins in the serum:
| Protein | Binding strength | Plasma concentration |
|---|---|---|
| thyroxine-binding globulin (TBG) | highest | lowest |
| transthyretin (TTR) | lower | higher |
| albumin | poorest | much higher |
In cerebrospinal fluid TTR is the primary carrier of T4. TTR also acts as a carrier ofretinol (vitamin A) through its association with retinol-binding protein (RBP) in the blood and the CSF. Less than 1% of TTR’s T4 binding sites are occupied in blood, which is taken advantage of below to prevent TTRs dissociation, misfolding and aggregation which leads to the degeneration of post-mitotic tissue.
Numerous other small molecules are known to bind in the thyroxine binding sites, including many natural products (such as resveratrol), drugs (Tafamidis,[2] or Vyndaqel, diflunisal,[3][4][5] flufenamic acid),[6] and toxins (PCB[7]).
Structure
TTR is a 55kDa homotetramer with a dimer of dimers quaternary structure that is synthesized in the liver, choroid plexus and retinal pigment epithelium for secretion into the bloodstream, cerebrospinal fluid and the eye, respectively. Each monomer is a 127-residue polypeptide rich in beta sheet structure. Association of two monomers via their edge beta-strands forms an extended beta sandwich. Further association of two of these dimers in a face-to-face fashion produces the homotetrameric structure and creates the two thyroxine binding sites per tetramer. This dimer-dimer interface, comprising the two T4 binding sites, is the weaker dimer-dimer interface and is the one the comes apart first in the process of tetramer dissociation.[8]
- Prealbumin at the US National Library of Medicine Medical Subject Headings (MeSH)
- ^ a b Razavi H, Palaninathan SK, Powers ET, Wiseman RL, Purkey HE, Mohamedmohaideen NN, Deechongkit S, Chiang KP, Dendle MT, Sacchettini JC, Kelly JW (June 2003). “Benzoxazoles as transthyretin amyloid fibril inhibitors: synthesis, evaluation, and mechanism of action”. Angew. Chem. Int. Ed. Engl. 42 (24): 2758–61.doi:10.1002/anie.200351179. PMID 12820260.
- ^ Sekijima Y, Dendle MA, Kelly JW (December 2006). “Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis”. Amyloid 13 (4): 236–49. doi:10.1080/13506120600960882.PMID 17107884.
- ^ Adamski-Werner SL, Palaninathan SK, Sacchettini JC, Kelly JW (January 2004). “Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis”. J. Med. Chem. 47 (2): 355–74. doi:10.1021/jm030347n.PMID 14711308.
- ^ Vilaro M, Arsequell G, Valencia G, Ballesteros A, Barluenga J, Nieto J, Planas A, Almeida R, Saraiva MJ (2007). “Reengineering TTR amyloid inhibition properties of diflunisal”. In Seldin DC, Skinner M, Berk JL, Connors LH. XIth International Symposium on Amyloidosis. Boca Raton: CRC.doi:10.1201/9781420043358.ch69. ISBN 1-4200-4281-5.
- ^ Baures PW, Oza VB, Peterson SA, Kelly JW (July 1999). “Synthesis and evaluation of inhibitors of transthyretin amyloid formation based on the non-steroidal anti-inflammatory drug, flufenamic acid”. Bioorg. Med. Chem. 7 (7): 1339–47.doi:10.1016/S0968-0896(99)00066-8. PMID 10465408.
- ^ Purkey HE, Palaninathan SK, Kent KC, Smith C, Safe SH, Sacchettini JC, Kelly JW (December 2004). “Hydroxylated polychlorinated biphenyls selectively bind transthyretin in blood and inhibit amyloidogenesis: rationalizing rodent PCB toxicity”.Chem. Biol. 11 (12): 1719–28.doi:10.1016/j.chembiol.2004.10.009. PMID 15610856.
- ^ Foss TR, Wiseman RL, Kelly JW (November 2005). “The pathway by which the tetrameric protein transthyretin dissociates”. Biochemistry 44 (47): 15525–33.doi:10.1021/bi051608t. PMID 16300401.

Semisynthetic Latrunculin Derivatives as Inhibitors of Metastatic Breast Cancer: Biological Evaluations, Preliminary Structure–Activity Relationship and Molecular Modeling Studies
The microfilament cytoskeleton protein actin plays an important role in cell biology and affects cytokinesis, morphogenesis, and cell migration. These functions usually fail and become abnormal in cancer cells. The marine-derived macrolides latrunculins A and B, from the Red Sea sponge Negombata magnifica, are known to reversibly bind actin monomers, forming 1:1 stoichiometric complexes with G-actin, disrupting its polymerization. To identify novel therapeutic agents for effective treatment of metastatic breast cancer, several semisynthetic derivatives of latrunculin A with diverse steric, electrostatic, and hydrogen bond donor and acceptor properties were rationally prepared. Analogues were designed to modulate the binding affinity toward G-actin. Examples of these reactions are esterification, acetylation, and N-alkylation. Semisynthetic latrunculins were then tested for their ability to inhibit pyrene-conjugated actin polymerization, and subsequently assayed for their antiproliferative and anti-invasive properties against MCF7 and MDA-MB-231 cells using MTT and invasion assays, respectively.
Mohammad A. Khanfar, Diaa T. A. Youssef and Khalid A. El Sayed
Article first published online: 30 DEC 2009 | DOI: 10.1002/cmdc.200900430
ChemMedChem
Volume 5, Issue 2, pages 274–285, February 1, 2010
Negombata magnifica, a Red Sea sponge (background), is the natural source of latrunculin A. A series of latrunculin A derivatives were synthesized and tested for their ability to inhibit G-actin polymerization and breast cancer cell proliferation and invasion. Molecular modeling simulations (inset) were applied to improve the understanding of the SAR of latrunculins.
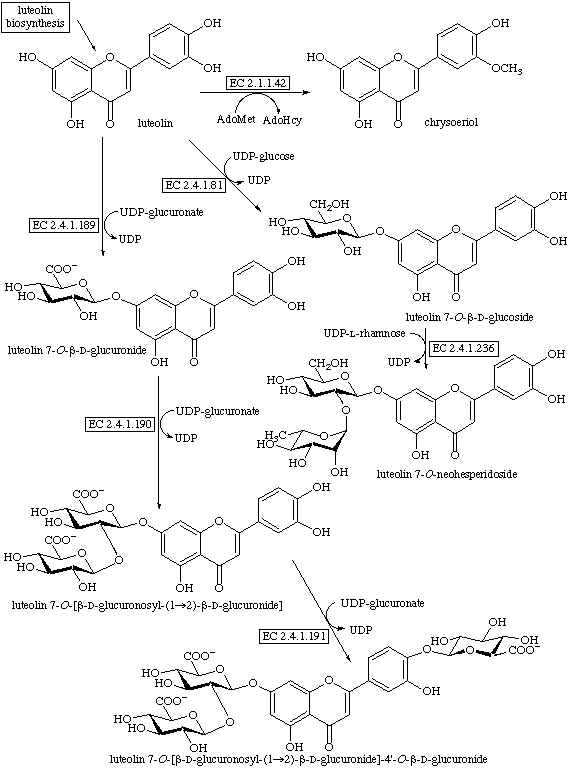
Study shows flavonoid Luteolin can block cancer cell signaling

Luteolin, a flavonoid compound commonly found in fruit and vegetables, has been found to be able to surppress the activity of cell signaling pathways (IGF and PI3K) that play key roles in growth of cancer cells.

LUTEOLIN
The study, published in BioMed Central’s open access journal BMC Gastroenterology, suggested the possibility of developing novel therapies based on the plant flavonoid Luteolin against colon cancer, the second most frequent cause of cancer-related death in the Western World. Colon cancer cells have elevated levels of IGF-II compared to normal colon tissues.
Luteolin, commonly found in green peppers, carrots, olive oil, rosemary, peppermint, oranges and celery, has been shown by preclinical studies to have anti-inflammatory, anti-oxidant, antimicrobial, and anticancer activities. Earlier studies have found that luteolin could inhibit angiogenesis, induce apoptosis and affect carcinogenesis in animal models, suggesting the possibility to use this flavonoid as cancer chemopreventive and chemotherapeutic agent.
A group of Korean scientist performed studies that show that luteolin inhibits the secretion of IGF-II by colon cancer cells and within two hours decreased the amount of receptor (IGF-IR) precursor protein. Luteolin also reduced the amount of active receptor (measured by IGF-I dependent phosphorylation).
It is noted in the publication that luteolin “downregulates the activation of the PI3K/Akt and ERK1/2 pathways via a reduction in IGF-IR signaling in HT-29 cells; this may be one of the mechanisms responsible for the observed luteolin-induced apoptosis and cell cycle arrest”.
Colon cancer cells have elevated levels of IGF-II compared to normal colon tissues. It is thought that this is part of the mechanism driving uncontrolled cell division and cancer growth.
Prof Jung Han Yoon Park, the corresponding author of the publication, says “Our study, showing that luteolin interferes with cell signaling in colon cancer cells, is a step forward in understanding how this flavonoid works. A fuller understanding of the in vivo results is essential to determine how it might be developed into an effective chemopreventive agent”.
Luteolin is a yellow crystalline compound. It is a flavonoid; to be specific, it is one of the more common flavones.[1] From preliminary research, it is thought to play a role in the human body possibly as an antioxidant, a free radical scavenger, a promoter ofcarbohydrate metabolism, or an immune system modulator.[citation needed] If applicable to the human condition, these characteristics may inhibit cancer mechanisms. Basic research results indicate luteolin as an anti-inflammatory agent,[2] with other potential effects on septic shock.[citation needed] It has been suggested for multiple sclerosis on the basis of in vitro work.[3]
Luteolin acts as a monoamine transporter activator, and is one of the few chemicals demonstrated to possess this property.[4]
Natural occurrences
Luteolin can be found in Terminalia chebula. It is most often found in leaves, but it is also seen in rinds, barks, clover blossom, and ragweed pollen.[1] It has also been isolated from Salvia tomentosa.[5]
In food
Dietary sources include celery, green pepper, parsley, thyme, dandelion, perilla,chamomile tea, carrots, olive oil, peppermint, rosemary, navel oranges, and oregano.[6][7]
It can also be found in the seeds of the palm Aiphanes aculeata.[8]

- Mann, John (1992). Secondary Metabolism (2nd ed.). Oxford, UK: Oxford University Press. pp. 279–280. ISBN 0-19-855529-6.
- Johnson; Kelley, KW; Johnson, RW (May 2008). “Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1”. Proc. Natl. Acad. Sci. U.S.A. 105 (21): 7534–9. doi:10.1073/pnas.0802865105. PMC 2396685.PMID 18490655.
- Theoharides (2009). “Luteolin as a Therapeutic Option for Multiple Sclerosis”. Journal of Neuroinflammation 6 (1): 29.doi:10.1186/1742-2094-6-29. PMC 2768692. PMID 19825165.
- Zhao, G; Qin, GW; Wang, J; Chu, WJ; Guo, LH (2010). “Functional activation of monoamine transporters by luteolin and apigenin isolated from the fruit of Perilla frutescens (L.) Britt”. Neurochemistry international 56 (1): 168–76. doi:10.1016/j.neuint.2009.09.015.PMID 19815045.
- A. Ulubelen, M. Miski, P. Neuman, and T. J. Mabry (1979). “Flavonoids of Salvia tomentosa (Labiatae)”. Journal of Natural Products 42(4): 261–3. doi:10.1021/np50003a002.
- Kayoko Shimoi, Hisae Okada, Michiyo Furugori, Toshinao Goda, Sachiko Takase, Masayuki Suzuki, Yukihiko Hara, Hiroyo Yamamoto, Naohide Kinae (1998). “Intestinal absorption of luteolin and luteolin 7-O-[beta]-glucoside in rats and humans”. FEBS Letters 438 (3): 220–4. doi:10.1016/S0014-5793(98)01304-0. PMID 9827549.
- López-Lázaro M. (2009). “Distribution and biological activities of the flavonoid luteolin“. Mini Rev Med Chem. 9 (1): 31–59.doi:10.2174/138955709787001712. PMID 19149659.
- Lee, D; Cuendet, M; Vigo, JS; Graham, JG; Cabieses, F; Fong, HH; Pezzuto, JM; Kinghorn, AD (2001). “A novel cyclooxygenase-inhibitory stilbenolignan from the seeds of Aiphanes aculeata”. Organic letters 3 (14): 2169–71. PMID 11440571.
Single molecule fights heart disease on two fronts
 1-Fe
1-FeIndian U-turn on diabetes drug ban

Pioglitazone increases the body’s sensitivity to insulin
Suspension of cheap and popular medicine reversed but will now come with new safety warnings
http://www.rsc.org/chemistryworld/2013/08/india-u-turn-diabetes-pioglitazone-drug-ban
Roche’s new skin cancer drug now available in UK
AUGUST 20, 2013
UK patients with a certain type of skin cancer can now get access to Roche’s once-daily pill Erivedge following its launch in the country.
Erivedge(vismodegib)胶囊
Erivedge (vismodegib) is the first medicine available in the UK for the treatment of patients with symptomatic metastatic basal cell carcinoma (BCC) or locally advanced BCC that is unsuitable for surgery or radiotherapy.
http://www.pharmatimes.com/Article/13-08-20/Roche_s_new_skin_cancer_drug_now_available_in_UK.aspx

Erivedge(vismodegib)胶囊
Brain Cancer Survival Improved Following FDA Approval of Bevacizumab, Mayo Study Finds

ROCHESTER, Minn. — A new population-based study has found that patients with glioblastoma who died in 2010, after the Food and Drug Administration (FDA) approval of bevacizumab, had lived significantly longer than patients who died of the disease in 2008, prior to the conditional approval of the drug for the treatment of the deadly brain cancer. Bevacizumab is used to treat patients with certain cancers whose cancer has spread. The study appears in the journal Cancer
http://www.pharmalive.com/study-brain-cancer-survival-improved-following-fda-approval-of-avastin
-Molecular-diagram.jpg)
STR REF –http://www.kidneycancerinstitute.com/Bevacizumab.html
IN CASE U NEED TO CONTACT ME THEN MAIL ME. amcrasto@gmail.com
Bevacizumab (trade name Avastin, Genentech/Roche) is an angiogenesis inhibitor, a drug that slows the growth of new blood vessels. It is licensed to treat various cancers, including colorectal, lung, breast (outside the USA), glioblastoma (USA only), kidney and ovarian.
Bevacizumab is a humanized monoclonal antibody that inhibits vascular endothelial growth factor A (VEGF-A). VEGF-A is a chemical signal that stimulates angiogenesis in a variety of diseases, especially in cancer. Bevacizumab was the first clinically availableangiogenesis inhibitor in the United States.

Bevacizumab was approved by the U.S. Food and Drug Administration (FDA) for certainmetastatic cancers. It received its first approval in 2004, for combination use with standardchemotherapy for metastatic colon cancer.It has since been approved for use in certain lung cancers, renal cancers, and glioblastoma multiforme of the brain.
At one point bevacizumab was approved for breast cancer by the FDA, but the approval was revoked on 18 November 2011. The approval for breast cancer was revoked because, although there was evidence that it slowed progression of metastatic breast cancer, there was no evidence that it extended life or improved quality of life, and it caused adverse effects including severe high blood pressure and hemorrhaging. In 2008, the FDA gave bevacizumab provisional approval for metastatic breast cancer, subject to further studies. The FDA’s advisory panel had recommended against approval. In July 2010, after new studies failed to show a significant benefit, the FDA’s advisory panel recommended against the indication for advanced breast cancer. Genentech requested a hearing, which was granted in June 2011. The FDA ruled to withdraw the breast cancer indication in November 2011. FDA approval is required for Genentech to market a drug for that indication. Doctors may sometimes prescribe it for that indication, although insurance companies are less likely to pay for it. The drug remains approved for breast cancer use in other countries including Australia.
Clinical trials are underway for many other indications including ovarian cancer, pediatric osteosarcoma, and certain non-malignant eye diseases. In the curative setting (adjuvant therapy), clinical studies are underway in breast cancer and lung cancer.

Actavis Receives Approval from French Competition Authority for Pending Warner Chilcott Acquisition

PARSIPPANY, N.J. and DUBLIN, IRELAND – August 9, 2013 – Actavis, Inc. (NYSE: ACT) and Warner Chilcott plc (NASDAQ: WCRX) today announced that they have received approval from the French Competition Authority for Actavis’ pending acquisition of Warner Chilcott. The companies previously received approval from the German Federal Cartel Office and have now received all ex-U.S. antitrust clearances required to complete the transaction.
read all at
http://www.pharmalive.com/french-authorities-approve-actavis-acquisition-of-warner-chilcott
………………………
………..
Bethlehem
 |
|
|---|---|
 |
|
AZATHIOPRINE
generic licensing news
http://newsletter.lead-doctors.com/ef1/preview_campaign.php?lf1=932543337a665012625317e9856877
Azathioprine is a chemotherapy drug, now rarely used for chemotherapy but more for immunosuppression in organ transplantation and autoimmune disease such as rheumatoid arthritis or inflammatory bowel disease or Crohn’s disease. It is a pro-drug, converted in the body to the active metabolite 6-mercaptopurine. Azathioprine acts to inhibit purine synthesis necessary for the proliferation of cells, especially leukocytes and lymphocytes. It is a safe and effective drug used alone in certain autoimmune diseases, or in combination with other immunosuppressants in organ transplantation.
Click here to contact Douglas Pharmaceuticals about this product.

Azathioprine was synthesized by George Herbert Hitchings and Gertrude Elion in 1957 (named BW 57-322) to produce 6-mercaptopurine (6-MP) in a metabolically active but masked form, and at first used as a chemotherapy drug.
Robert Schwartz investigated the effect of 6-MP on the immune response in 1958 and discovered that it profoundly suppresses the formation of antibodies when given to rabbits together with antigens. Following the work done by Sir Peter Medawar and Gertrude Elion in discovering the immunological basis of rejection of transplanted tissues and organs, and Schwartz’s researches on 6-MP, Sir Roy Calne, the British pioneer in transplantation, introduced 6-MP as an experimental immunosuppressant for kidney and heart transplants. When Calne asked Elion for related compounds to investigate, she suggested azathioprine, which was subsequently found out to be superior (as effective and less toxic to the bone marrow) by Calne. On 5 April 1962, with regimens consisting of azathioprine and prednisone, the transplantation of kidneys to unrelated recipients (allotransplantation) was successful for the first time.For many years, this kind of dual therapy with azathioprine and glucocorticoids was the standard antirejection regimen, until ciclosporin was introduced into clinical practice (by Calne as well) in 1978.Ciclosporin has now replaced some of the azathioprine use due to a longer survival time, especially in heart-related transplantations.Moreover, despite being considerably more expensive, mycophenolate mofetil is also increasingly being used in place of azathioprine in organ transplantation, as it is associated with less bone marrow suppression, fewer opportunistic infections, and a lower incidence of acute rejection
Azathioprine is a thiopurine linked to a second heterocycle (an imidazole derivative) via a thioether. It is a pale yellow solid with a slightly bitter taste and a melting point of 238–245 °C. It is practically insoluble in water and only slightly soluble in lipophilic solvents such as chloroform, ethanol and diethylether. It dissolves in alkaline aqueous solutions, where it hydrolyzes to 6-mercaptopurine.
Azathioprine is synthesized from 5-chloro-1-methyl-4-nitro-1H-imidazole and 6-mercaptopurine in dimethyl sulfoxide (DMSO). The synthesis of the former starts with an amide from methylamine and diethyl oxalate, which is then cyclizised and chlorinated with phosphorus pentachloride; the nitro group is introduced with nitric and sulfuric acid.

Azathioprine (INN, /ˌæzəˈθaɪɵpriːn/, abbreviated AZA) is an immunosuppressive drug used in organ transplantation and autoimmune diseases and belongs to the chemical class of purine analogues.[1] Synthesized originally as a cancer drug and a prodrug for mercaptopurine in 1957, it has been widely used as an immunosuppressant for more than 50 years.[2]
Azathioprine acts as a prodrug for mercaptopurine, inhibiting an enzyme that is required for the synthesis of DNA. Thus it most strongly affects proliferating cells, such as the T cells and B cells of the immune system.[3][4]
The main adverse effect of azathioprine is bone marrow suppression, which can be life-threatening, especially in people with a genetic deficiency of the enzyme thiopurine S-methyltransferase.[5] It is also listed by the International Agency for Research on Cancer as a Group 1 carcinogen (carcinogenic to humans).[6]
Azathioprine is produced by a number of manufacturers under different brand names (Azasan by Salix in the U.S., Imuran by GlaxoSmithKline in Canada, the U.S., Australia, Ireland and Great Britain, Azamun in Finland and Imurel in Scandinavia and France, among others).
Azathioprine is used alone or in combination with other immunosuppressive therapy to prevent rejection following organ transplantation, and to treat an array of autoimmune diseases, including rheumatoid arthritis, pemphigus, systemic lupus erythematosus, Behçet’s disease and other forms of vasculitis, autoimmune hepatitis, atopic dermatitis, myasthenia gravis, neuromyelitis optica (Devic’s disease), restrictive lung disease, and others. It is also an important therapy and steroid-sparing agent for inflammatory bowel disease (such as Crohn’s disease and ulcerative colitis) and for multiple sclerosis, which are immune-mediated as well.
In the United States it is currently approved by the Food and Drug Administration (FDA) for use in kidney transplantation from human donors, and for rheumatoid arthritis. Other uses are off-label.
- Azathioprine – FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Azathioprine – Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Azathioprine – Detailed information from DrugBank.


















