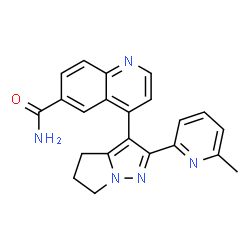
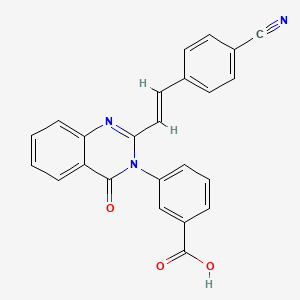
Galunisertib
Phase III
A TGF-beta receptor type-1 inhibitor potentially for the treatment of myelodysplastic syndrome (MDS) and solid tumours.

LY-2157299
CAS No.700874-72-2
4-[2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline-6-carboxamide
6-Quinolinecarboxamide, 4-[5,6-dihydro-2-(6-methyl-2-pyridinyl)-4H-pyrrolo[1,2-b]pyrazol-3-yl]-
700874-72-2
- Molecular FormulaC22H19N5O
- Average mass369.419 Da


4-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl)quinoline-6-carboxamide
4-(2-(6-Methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl)quinolin-6-carboxamide monohydrate
Anal. Calcd for C22H19N5O·H2O: C, 68.20; H, 5.46; N, 18.08. Found: C, 68.18; H, 5.34; N, 17.90.
1H NMR (DMSO-d6: δ) 1.74 (s, 3H), 2.63 (m, 2H), 2.82 (br s, 2H), 4.30 (t, J = 7.2 Hz, 2H), 6.93 (m, 1H), 7.37 (s, 1H), 7.41 (d, J = 4.4 Hz, 1H), 7.56 (m, 1H), 7.58 (m, 1H), 8.04, (s, 1H), 8.04 (d, J = 4.4 Hz, 1H), 8.12 (dd, J = 8.8, 1.6 Hz, 1H), 8.25 (d, J = 2.0 Hz, 1H), 8.87 (d, J = 4.4 Hz, 1H).
13C NMR (DMSO-d6: δ) 22.56, 23.24, 25.58, 48.01, 109.36, 117.74, 121.26, 122.95, 126.73, 127.16 (2C), 129.01, 131.10, 136.68, 142.98, 147.20, 148.99, 151.08, 151.58, 152.13, 156.37, 167.47.
IR (KBr): 3349, 3162, 3067, 2988, 2851, 1679, 1323, 864, 825 cm–1.
HRMS (m/z M + 1): Calcd for C22H19N5O: 370.1653. Found: 370.1662.
GalunisertibAn orally available, small molecule antagonist of the tyrosine kinase transforming growth factor-beta (TGF-b) receptor type 1 (TGFBR1), with potential antineoplastic activity. Upon administration, galunisertib specifically targets and binds to the kinase domain of TGFBR1, thereby preventing the activation of TGF-b-mediated signaling pathways. This may inhibit the proliferation of TGF-b-overexpressing tumor cells. Dysregulation of the TGF-b signaling pathway is seen in a number of cancers and is associated with increased cancer cell proliferation, migration, invasion and tumor progression.
 .
.
- OriginatorEli Lilly
- DeveloperEli Lilly; National Cancer Institute (USA); Vanderbilt-Ingram Cancer Center; Weill Cornell Medical College
- ClassAntineoplastics; Pyrazoles; Pyridines; Pyrroles; Quinolines; Small molecules
- Mechanism of ActionPhosphotransferase inhibitors; Transforming growth factor beta1 inhibitors
-
- Phase II/IIIMyelodysplastic syndromes
- Phase IIBreast cancer; Glioblastoma; Hepatocellular carcinoma
- Phase I/IIGlioma; Non-small cell lung cancer; Pancreatic cancer
- Phase ICancer; Solid tumours
Most Recent Events
- 26 Apr 2016Eli Lilly plans a pharmacokinetics phase I trial in Healthy volunteers in United Kingdom (PO) (NCT02752919)
- 16 Apr 2016Pharmacodynamics data from a preclinical study in Cancer presented at the 107th Annual Meeting of the American Association for Cancer Research (AACR-2016)
- 06 Apr 2016Eli Lilly and AstraZeneca plan a phase Ib trial for Pancreatic cancer (Second-line therapy or greater, Metastatic disease, Recurrent, Combination therapy) in USA, France, Italy, South Korea and Spain (PO) (NCT02734160)
Transforming growth factor-beta (TGF-β) signaling regulates a wide range of biological processes. TGF-β plays an important role in tumorigenesis and contributes to the hallmarks of cancer, including tumor proliferation, invasion and metastasis, inflammation, angiogenesis, and escape of immune surveillance. There are several pharmacological approaches to block TGF-β signaling, such as monoclonal antibodies, vaccines, antisense oligonucleotides, and small molecule inhibitors. Galunisertib (LY2157299 monohydrate) is an oral small molecule inhibitor of the TGF-β receptor I kinase that specifically downregulates the phosphorylation of SMAD2, abrogating activation of the canonical pathway. Furthermore, galunisertib has antitumor activity in tumor-bearing animal models such as breast, colon, lung cancers, and hepatocellular carcinoma. Continuous long-term exposure to galunisertib caused cardiac toxicities in animals requiring adoption of a pharmacokinetic/pharmacodynamic-based dosing strategy to allow further development. The use of such a pharmacokinetic/pharmacodynamic model defined a therapeutic window with an appropriate safety profile that enabled the clinical investigation of galunisertib. These efforts resulted in an intermittent dosing regimen (14 days on/14 days off, on a 28-day cycle) of galunisertib for all ongoing trials. Galunisertib is being investigated either as monotherapy or in combination with standard antitumor regimens (including nivolumab) in patients with cancer with high unmet medical needs such as glioblastoma, pancreatic cancer, and hepatocellular carcinoma. The present review summarizes the past and current experiences with different pharmacological treatments that enabled galunisertib to be investigated in patients.
| Company |
Eli Lilly and Co. |
| Description |
Transforming growth factor (TGF) beta receptor 1 (TGFBR1; ALK5) inhibitor |
| Molecular Target |
Transforming growth factor (TGF) beta receptor 1 (TGFBR1) (ALK5) |
| Mechanism of Action |
Transforming growth factor (TGF) beta 1 inhibitor |
| Therapeutic Modality |
Small molecule |
Bristol-Myers Squibb and Lilly Enter Clinical Collaboration Agreement to Evaluate Opdivo (nivolumab) in Combination with Galunisertib in Advanced Solid Tumors

NEW YORK & INDIANAPOLIS–(BUSINESS WIRE)– Bristol-Myers Squibb Company (NYSE:BMY) and Eli Lilly and Company (NYSE:LLY) announced today a clinical trial collaboration to evaluate the safety, tolerability and preliminary efficacy of Bristol-Myers Squibb’s immunotherapy Opdivo (nivolumab) in combination with Lilly’s galunisertib (LY2157299). The Phase 1/2 trial will evaluate the investigational combination of Opdivo and galunisertib as a potential treatment option for patients with advanced (metastatic and/or unresectable) glioblastoma, hepatocellular carcinoma and non-small cell lung cancer.
Opdivo is a human programmed death receptor-1 (PD-1) blocking antibody that binds to the PD-1 receptor expressed on activated T-cells. Galunisertib (pronounced gal ue” ni ser’tib) is a TGF beta R1 kinase inhibitor that in vitro selectively blocks TGF beta signaling. TGF beta promotes tumor growth, suppresses the immune system and increases the ability of tumors to spread in the body. This collaboration will address the hypothesis that co-inhibition of PD-1 and TGF beta negative signals may lead to enhanced anti-tumor immune responses than inhibition of either pathway alone.
“Advanced solid tumors represent a serious unmet medical need among patients with cancer,” said Michael Giordano, senior vice president, Head of Development, Oncology, Bristol-Myers Squibb. “Our clinical collaboration with Lilly underscores Bristol-Myers Squibb’s continued commitment to explore combination regimens from our immuno-oncology portfolio with other mechanisms of action that may accelerate the development of new treatment options for patients.”
“Combination therapies will be key to addressing tumor heterogeneity and the inevitable resistance that is likely to develop to even the most promising new tailored therapies,” said Richard Gaynor, M.D., senior vice president, Product Development and Medical Affairs, Lilly Oncology. “To that end, having multiple cancer pathways and technology platforms will be critical in an era of combinations to ensure sustainability beyond any single asset.”
The study will be conducted by Lilly. Additional details of the collaboration were not disclosed.
About Galunisertib
Galunisertib (pronounced gal ue” ni ser’tib) is Lilly’s TGF beta R1 kinase inhibitor that in vitro selectively blocks TGF beta signaling. TGF beta promotes tumors growth, suppresses the immune system, and increases the ability of tumors to spread in the body.
Immune function is suppressed in cancer patients, and TGF beta worsens immunosuppression by enhancing the activity of immune cells called T regulatory cells. TGF beta also reduces immune proteins, further decreasing immune activity in patients
Galunisertib is currently under investigation as an oral treatment for advanced/metastatic malignancies, including Phase 2 evaluation in hepatocellular carcinoma, myelodysplastic syndromes (MDS), glioblastoma, and pancreatic cancer.
PATENT
WO 2004048382
The disclosed invention also relates to the select compound of Formula II:
Formula II
2-(6-methyl-pyridin-2-yI)-3-[6-amido-quinolin-4-yl)-5,6-dihydro-4H-pyrrolo[l,2- bjpyrazole and the phannaceutically acceptable salts thereof.
The compound above is genetically disclosed and claimed in PCT patent application PCT/US02/11884, filed 13 May 2002, which claims priority from U.S. patent application U. S . S .N. 60/293 ,464, filed 24 May 2001 , and incorporated herein by reference. The above compound has been selected for having a surprisingly superior toxicology profile over the compounds specifically disclosed in application cited above.
The following scheme illustrates the preparation of the compound of Formula II.
Scheme II
Cs2C03
The following examples further illustrate the preparation of the compounds of this invention as shown schematically in Schemes I and II. Example 1
Preparation of 7-(2-morpholin-4-yI-ethoxy)-4-(2-pyridin-2-yl-5,6-dihydro-4H- pyrroIo[l,2-b]pyrazol-3-yl)-q inoline
A. Preparation of 4-(2-pyridin-2-yl-5,6-dihydro-4H-pyrrolo[l,2-b]pyrazol-3-yl)- 7-[2-(tetrahydropyran-2-yIoxy)ethoxy]quinoIine
Heat 4-(2-pyridm-2-yl-5,6-dihydro-4H-pyrrolo[l,2-b]pyrazol-3-yl)-quinolin-7-ol (376 mg, 1.146 mmol), cesium carbonate (826 mg, 2.54 mmol), and 2-(2- bromoethoxy)tetrahydro-2H-pyran (380 μL, 2.52 mmol) in DMF (5 mL) at 120 °C for 4 hours. Quench the reaction with saturated sodium chloride and then extract with chloroform. Dry the organic layer over sodium sulfate and concentrate in vacuo. Purify the reaction mixture on a silica gel column eluting with dichloromethane to 10% methanol in dichloromethane to give the desired subtitled intermediate as a yellow oil (424 mg, 81%). MS ES+m/e 457.0 (M+l).
EXAMPLE 2
Preparation of 2-(6-methyl-pyridin-2-yl)-3-[6-amido-quinolin-4-yl)-5,6-dihydro-4H-pyrrolo[l,2- b]pyrazole
A. Preparation of 6-bromo-4-methyI-quinoline
Stir a solution of 4-bromo-phenylamine (1 eq), in 1,4-dioxane and cool to approximately 12 °C. Slowly add sulfuric acid (2 eq) and heat at reflux. Add methyl vinyl ketone (1.5 eq) drop wise into the refluxing solution. Heat the solution for 1 hour after addition is complete. Evaporate the reaction solution to dryness and dissolve in methylene chloride. Adjust the solution to pH 8 with 1 M sodium carbonate and extract three times with water. Chromatograph the residue on SiO (70/30 hexane/ethyl acetate) to obtain the desired subtitled inteπnediate. MS ES+ m e = 158.2 (M+l). B. Preparation of 6-methyl-pyridine-2-carboxylic acid methyl ester
Suspend 6-methyl-pyridine-2-carboxylic acid (10 g, 72.9 mmol) in methylene chloride (200 mL). Cool to 0 °C. Add methanol (10 mL), 4-dimethylaminopyridine (11.6 g, 94.8 mmol), and l-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC)
(18.2 g, 94.8 mmol). Stir the mixture at room temperature for 6 hours, wash with water and brine, and dry over sodium sulfate. Filter the mixture and concentrate in vacuo.
Chromatograph the residue on SiO2 (50% ethyl acetate/hexanes) to obtain the desired subtitled intermediate, 9.66 g (92%), as a colorless liquid. 1H NMR (CDC13) 6 7.93-7.88 (m, IH), 7.75-7.7 (m, IH), 7.35-7.3 (m, IH), 4.00 (s, 3H), 2.60 (s, 3H).
C. Preparation of 2-(6-bromo-quinoIin-4-yl)-l-(6-methyl-pyridin-2-yl)-ethanone Dissolve 6-bromo-4-methyl-quinoline (38.5 g, 153 mmol) in 600 mL dry THF.
Cool to -70° C and treat with the dropwise addition of 0.5 M potassium hexamethyldisilazane (KN(SiMe )2 (400 mL, 200 mmol) over 2 hours while keeping the temperature below -65 °C. Stir the resultant solution at -70°C for 1 hour and add a solution of 6-methylpyridine-2-carboxylic acid methyl ester (27.2, 180 mmol) in 100 mL dry THF dropwise over 15 minutes. During the addition, the mixture will turn from dark red to pea-green and form a precipitate. Stir the mixture at -70°C over 2 hours then allow it to warm to ambient temperature with stirring for 5 hours. Cool the mixture then quench with 12 N HC1 to pH=l . Raise the pH to 9 with solid potassium carbonate. Decant the solution from the solids and extract twice with 200 mL ethyl acetate. Combine the organic extracts, wash with water and dry over potassium carbonate. Stir the solids in 200 mL water and 200 mL ethyl acetate and treat with additional potassium carbonate. Separate the organic portion and dry with the previous ethyl acetate extracts. Concentrate the solution in vacuo to a dark oil. Pass the oil through a 300 mL silica plug with methylene chloride then ethyl acetate. Combine the appropriate fractions and concentrate in vacuo to yield an amber oil. Rinse the oil down the sides of the flask with methylene chloride then dilute with hexane while swirling the flask to yield 38.5 g (73.8 %) of the desired subtitled intermediate as a yellow solid. MS ES+ = 341 (M+l)v D. Preparation of l-[2-(6-bromo-quinolin-4-yI)-l-(6-methyl-pyridin-2-yl)- ethylideneamino]-pyrrolidin-2-one
Stir a mixture of 2-(6-bromo-quinolin-4-yl)-l-(6-methyl-pyridin-2-yl)-ethanone (38.5 g, 113 mmol) and 1-aminopyrrolidinone hydrochloride (20 g, 147 mmol) in 115 mL pyridine at ambient temperature for 10 hours. Add about 50 g 4 A unactivated sieves. Continue stirring an additional 13 h and add 10-15 g silica and filter the mixture through a 50 g silica plug. Elute the silica plug with 3 L ethyl acetate. Combine the filtrates and concentrate in vacuo. Collect the hydrazone precipitate by filtration and suction dry to yield 33.3 g (69.7%) of the desired subtitled intermediate as an off-white solid. MS ES+ = 423 (M+l).
E. Preparation of 6-bromo-4-[2-(6-methyl-pyridin-2-yι)-5,6-dihydro-4H- pyrrolo[l,2-b]pyrazol-3-yl]-quinoline
To a mixture of (1.2 eq.) cesium carbonate and l-[2-(6-bromo-qumolin-4-yl)-l- (6-methyl-pyridin-2-yl)-ethylideneamino]-pyrrolidin-2-one (33.3 g, 78.7 mmol) add 300 mL dry N,N-dimethylformamide. Stir the mixture 20 hours at 100°C. The mixture may turn dark during the reaction. Remove the N,N-dimethylformamide in vacuo. Partition the residue between water and methylene chloride. Extract the aqueous portion with additional methylene chloride. Filter the organic solutions through a 300 mL silica plug, eluting with 1.5 L methylene chloride, 1.5 L ethyl acetate and 1.5 L acetone. Combine the appropriate fractions and concentrate in vacuo. Collect the resulting precipitate by filtration to yield 22.7 g (71.2%) of the desired subtitled intermediate as an off-white solid. MS ES+ = 405 (M+l).
F. Preparation of 4-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-4H-pyrrolo[l,2- b]pyrazol-3-yl]-quinoline-6-carboxylic acid methyl ester
Add 6-bromo-4-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-4H-pyrrolo[l,2- b]pyrazol-3-yl]-quinoline (22.7 g, 45 mmol) to a mixture of sodium acetate (19 g, 230 mmol) and the palladium catalyst [1,1 ‘- bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane (1:1) (850 mg, 1.04 mmol) in 130 mL methanol. Place the mixture under 50 psi carbon monoxide atmosphere and stir while warming to 90° C over 1 hour and with constant charging with additional carbon monoxide. Allow the mixture to cool over 8 hours, recharge again with carbon monoxide and heat to 90 °C. The pressure may rise to about 75 PSI. The reaction is complete in about an hour when the pressure is stable and tic (1 : 1 toluene/acetone) shows no remaining bromide. Partition the mixture between methylene chloride (600 mL) and water (1 L). Extract the aqueous portion with an additional portion of methylene chloride (400 mL.) Filter the organic solution through a 300 mL silica plug and wash with 500 mL methylene chloride, 1200 mL ethyl acetate and 1500 mL acetone. Discard the acetone portion. Combine appropriate fractions and concentrate to yield 18.8 g (87.4%) of the desired subtitled intermediate as a pink powder. MS ES+ = 385 (M+l).
G. Preparation of 2-(6-methyl-pyridin-2-yl)-3-[6-amido-quinolin-4-yι)-5,6- dihydro-4H-pyrrolo[l,2-b]pyrazole
Warm a mixture of 4-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-4H-pyrrolo[l,2- b]pyrazol-3-yl]-quinolme-6-carboxylic acid methyl ester in 60 mL 7 N ammonia in methanol to 90 °C in a stainless steel pressure vessel for 66 hours. The pressure will rise to about 80 PSI. Maintain the pressure for the duration of the reaction. Cool the vessel and concentrate the brown mixture in vacuo. Purify the residual solid on two 12 g Redi- Pak cartridges coupled in series eluting with acetone. Combine appropriate fractions and concentrate in vacuo. Suspend the resulting nearly white solid in methylene chloride, dilute with hexane, and filter. The collected off-white solid yields 1.104 g (63.8%) of the desired title product. MS ES+ = 370 (M+l).
PAPER
http://pubs.acs.org/doi/abs/10.1021/op4003054
Application of Kinetic Modeling and Competitive Solvent Hydrolysis in the Development of a Highly Selective Hydrolysis of a Nitrile to an Amide
Lilly Research Laboratories, Eli Lilly and Company, Lilly Corporate Center, Indianapolis, Indiana 46285, United States
Org. Process Res. Dev., 2014, 18 (3), pp 410–416
DOI: 10.1021/op4003054
Publication Date (Web): February 11, 2014
Copyright © 2014 American Chemical Society
Abstract
A combination of mechanism-guided experimentation and kinetic modeling was used to develop a mild, selective, and robust hydroxide-promoted process for conversion of a nitrile to an amide using a substoichiometric amount of aqueous sodium hydroxide in a mixed water and N-methyl-2-pyrrolidone solvent system. The new process eliminated a major reaction impurity, minimized overhydrolysis of the product amide by selection of a solvent that would be sacrificially hydrolyzed, eliminated genotoxic impurities, and improved the intrinsic safety of the process by eliminating the use of hydrogen peroxide. The process was demonstrated in duplicate on a 90 kg scale, with 89% isolated yield and greater than 99.8% purity.
| Patent ID |
Date |
Patent Title |
| US2015289795 |
2015-10-15 |
METHODS AND KITS FOR THE PROGNOSIS OF COLORECTAL CANCER |
| US2014348889 |
2014-11-27 |
Compositions and Methods for Treating and Preventing Neointimal Stenosis |
| US2014328860 |
2014-11-06 |
METHODS FOR STIMULATING HEMATOPOIETIC RECOVERY BY INHIBITING TGF BETA SIGNALING |
| US2014127228 |
2014-05-08 |
INHIBITION OF TGFBETA SIGNALING TO IMPROVE MUSCLE FUNCTION IN CANCER |
| US2014128349 |
2014-05-08 |
ADMINISTERING INHIBITORS OF TGFBETA SIGNALING IN COMBINATION WITH BENZOTHIAZEPINE DERIVATIVES TO IMPROVE MUSCLE FUNCTION IN CANCER PATIENTS |
| US2013071931 |
2013-03-21 |
PROCESS FOR HEPATIC DIFFERENTIATION FROM INDUCED HEPATIC STEM CELLS, AND INDUCED HEPATIC PROGENITOR CELLS DIFFERENTIATED THEREBY |
| US7872020 |
2011-01-18 |
TGF-[beta] inhibitors |
| US7834029 |
2010-11-16 |
QUINOLINYL-PYRROLOPYRAZOLES |
| US7265225 |
2007-09-04 |
Quinolinyl-pyrrolopyrazoles |
REFERENCES
1: Rodón J, Carducci M, Sepulveda-Sánchez JM, Azaro A, Calvo E, Seoane J, Braña I, Sicart E, Gueorguieva I, Cleverly A, Pillay NS, Desaiah D, Estrem ST, Paz-Ares L, Holdhoff M, Blakeley J, Lahn MM, Baselga J. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-β receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest New Drugs. 2014 Dec 23. [Epub ahead of print] PubMed PMID: 25529192.
2: Kovacs RJ, Maldonado G, Azaro A, Fernández MS, Romero FL, Sepulveda-Sánchez JM, Corretti M, Carducci M, Dolan M, Gueorguieva I, Cleverly AL, Pillay NS, Baselga J, Lahn MM. Cardiac Safety of TGF-β Receptor I Kinase Inhibitor LY2157299 Monohydrate in Cancer Patients in a First-in-Human Dose Study. Cardiovasc Toxicol. 2014 Dec 9. [Epub ahead of print] PubMed PMID: 25488804.
3: Rodon J, Carducci MA, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, Brana I, Sicart E, Gueorguieva I, Cleverly AL, Sokalingum Pillay N, Desaiah D, Estrem ST, Paz-Ares L, Holdoff M, Blakeley J, Lahn MM, Baselga J. First-in-Human Dose Study of the Novel Transforming Growth Factor-β Receptor I Kinase Inhibitor LY2157299 Monohydrate in Patients with Advanced Cancer and Glioma. Clin Cancer Res. 2014 Nov 25. pii: clincanres.1380.2014. [Epub ahead of print] PubMed PMID: 25424852.
4: Huang C, Wang H, Pan J, Zhou D, Chen W, Li W, Chen Y, Liu Z. Benzalkonium Chloride Induces Subconjunctival Fibrosis Through the COX-2-Modulated Activation of a TGF-β1/Smad3 Signaling Pathway. Invest Ophthalmol Vis Sci. 2014 Nov 18;55(12):8111-22. doi: 10.1167/iovs.14-14504. PubMed PMID: 25406285.
5: Cong L, Xia ZK, Yang RY. Targeting the TGF-β receptor with kinase inhibitors for scleroderma therapy. Arch Pharm (Weinheim). 2014 Sep;347(9):609-15. doi: 10.1002/ardp.201400116. Epub 2014 Jun 11. PubMed PMID: 24917246.
6: Gueorguieva I, Cleverly AL, Stauber A, Sada Pillay N, Rodon JA, Miles CP, Yingling JM, Lahn MM. Defining a therapeutic window for the novel TGF-β inhibitor LY2157299 monohydrate based on a pharmacokinetic/pharmacodynamic model. Br J Clin Pharmacol. 2014 May;77(5):796-807. PubMed PMID: 24868575; PubMed Central PMCID: PMC4004400.
7: Oyanagi J, Kojima N, Sato H, Higashi S, Kikuchi K, Sakai K, Matsumoto K, Miyazaki K. Inhibition of transforming growth factor-β signaling potentiates tumor cell invasion into collagen matrix induced by fibroblast-derived hepatocyte growth factor. Exp Cell Res. 2014 Aug 15;326(2):267-79. doi: 10.1016/j.yexcr.2014.04.009. Epub 2014 Apr 26. PubMed PMID: 24780821.
8: Giannelli G, Villa E, Lahn M. Transforming growth factor-β as a therapeutic target in hepatocellular carcinoma. Cancer Res. 2014 Apr 1;74(7):1890-4. doi: 10.1158/0008-5472.CAN-14-0243. Epub 2014 Mar 17. Review. PubMed PMID: 24638984.
9: Dituri F, Mazzocca A, Peidrò FJ, Papappicco P, Fabregat I, De Santis F, Paradiso A, Sabbà C, Giannelli G. Differential Inhibition of the TGF-β Signaling Pathway in HCC Cells Using the Small Molecule Inhibitor LY2157299 and the D10 Monoclonal Antibody against TGF-β Receptor Type II. PLoS One. 2013 Jun 27;8(6):e67109. Print 2013. PubMed PMID: 23826206; PubMed Central PMCID: PMC3694933.
10: Bhola NE, Balko JM, Dugger TC, Kuba MG, Sánchez V, Sanders M, Stanford J, Cook RS, Arteaga CL. TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest. 2013 Mar 1;123(3):1348-58. doi: 10.1172/JCI65416. Epub 2013 Feb 8. PubMed PMID: 23391723; PubMed Central PMCID: PMC3582135.
11: Bhattachar SN, Perkins EJ, Tan JS, Burns LJ. Effect of gastric pH on the pharmacokinetics of a BCS class II compound in dogs: utilization of an artificial stomach and duodenum dissolution model and GastroPlus,™ simulations to predict absorption. J Pharm Sci. 2011 Nov;100(11):4756-65. doi: 10.1002/jps.22669. Epub 2011 Jun 16. PubMed PMID: 21681753.
12: Bueno L, de Alwis DP, Pitou C, Yingling J, Lahn M, Glatt S, Trocóniz IF. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer. 2008 Jan;44(1):142-50. Epub 2007 Nov 26. PubMed PMID: 18039567.

References
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4539082/
http://www.ncbi.nlm.nih.gov/pubmed/26057634
https://clinicaltrials.gov/ct2/show/NCT0242334
Bhattachar, Shobha N.; Journal of Pharmaceutical Sciences 2011, 100(11), 4756-4765
Investigational new drugs (2015), 33(2), 357-70.
//////////TGF-β, TGF-βRI kinase inhibitor, ALK5, galunisertib, LY2157299, cancer, clinical trials, PHASE 3
CC1=CC=CC(=N1)C2=NN3CCCC3=C2C4=C5C=C(C=CC5=NC=C4)C(=O)N




































.jpg?n=3254)






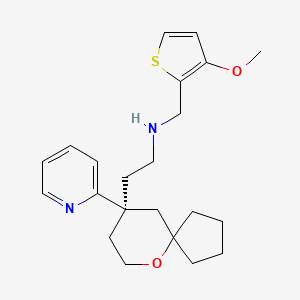
























 .
.






