In the Pharmacopoeial Forum (PF) 42(3) (May-June 2016) the USP General Chapters – Packaging and Distribution Expert Committee proposes a new general chapter <661.3> Plastic Components and Systems Used in Pharmaceutical Manufacturing and a revised version of general chapter <1661> Evaluation of Plastic Packaging and Manufacturing Systems and Their Materials of construction with Respect to Their User Safety Impact. Read more about USPs Proposal on Plastic Components and Systems Used in Pharmaceutical Manufacturing.
<1661> Evaluation of Plastic Packaging and Manufacturing Systems and Their Materials of construction with Respect to Their User Safety Impact. Read more about USPs Proposal on Plastic Components and Systems Used in Pharmaceutical Manufacturing.
see
In the Pharmacopoeial Forum (PF) 42(3) (May-June 2016) the USP General Chapters – Packaging and Distribution Expert Committee proposes a new chapter to address the qualification of plastic components used in the manufacture of APIs (pharmaceutical and biopharmaceutical) and drug products (DPs). The proposed Title of the new chapter <661.3> is Plastic Components and Systems Used in Pharmaceutical Manufacturing. The draft is open for comment until July 31, 2016.
The chapter is part of a suite of chapters, including Plastic Packaging Systems and Their Materials of Construction <661>,Plastic Materials of Construction <661.1>, Plastic Packaging Systems for Pharmaceutical Use <661.2>, and Evaluation of Plastic Packaging and Manufacturing Systems and Their Materials of construction with Respect to Their User Safety Impact<1661>. In addition a section has been added to general chapter <1661> to support the use and understanding of the new general chapter <661.3>. The revision of general chapter <1661> (including change of title) also appears in the PF issue 42(3).
The chapter <661.3> addresses the qualification of plastic components used in pharmaceutical manufacturing and is applicable solely to those processes that involve liquid process streams and process intermediates due to the expected increased degree of interaction with liquids. Plastic manufacturing systems for pharmaceutical use include – for example – bags, cassettes, chromatographic columns, connectors, filling needles, filters, sensors, tanks, tubing, and valves.Elastomeric parts such as diaphragms, gaskets, and O-rings are not in the scope of this chapter. A flow diagram that shows a typical bioprocess DP production suite is shown in general chapter <1661>, Figure 2.
The manufacturer of APIs and DPs is responsible for ensuring that the plastic components and systems used are suited for the intended purpose. It is likely that raw materials, intermediates, process streams, APIs, and DPs will get in contact with one or more plastic component(s) of the manufacturing suite during the manufacturing process, resulting in process-related impurities (PrIs). PrIs have the potential to alter a quality attribute of the DP, if the PrIs persist through the manufacturing process.
Plastic manufacturing components and systems are chemically suited for their intended use with respect to safety if:
- they are constructed from well-characterized materials that have been intentionally chosen for use as established by the test methods included in general chapter <661.1>;
- The general physicochemical properties of the components have been established;
- The biocompatibility (biological reactivity) has been appropriately established;
- They have been established as safe by means of the appropriate chemical testing, such as extractables or leachables profiling and toxicological assessment of the test data (“chemical safety assessment”).
The chapter provides guidance on the appropriate application of biological reactivity tests (reference to general chapters <87>, <88>) and physicochemical tests (reference to Food Additive regulations and general chapter <661.1>, where applicable) for manufacturing components and systems. A two-stage approach consisting of an Initial Assessment followed by a Risk assessment leads to the required level of component characterization. The Initial Assessment examines the factors present for demonstration of equivalence with a comparator component or system by looking at the following parameters:
- purpose and composition of component or system;
- composition of DP(s);
- processing conditions;
- product dosage form.
The demonstration of equivalence would allow acceptance of the component (or system) without any further characterization. If equivalence cannot be established between the component (or system) under consideration and the comparator, then a Risk Assessment should be conducted. The risk assessment matrix is provided in detail in general chapter <1661>. The outcome of this assessment results in three risk levels: low (A), moderate (B), and high (C). These levels are linked according to the risk of the individual dosage form (e.g. solid oral and liquid oral, others than solid oral and liquid oral) to test requirements as shown in the draft chapter <661.3>. All three risk levels require identification of the component or system as specified in general chapter <661.1>. Identity is only required for those components or systems that consist of single materials of construction (individual polymers only). Biological reactivity testing according to USP general chapter <87> (In Vitro) is required for all levels plus testing according to Class VI in <88> (In Vivo) for Level B and C. Level A and B require that the component or system be tested as specified in general chapter <661.1> for physicochemical characteristics and extractable metals characteristics. Level C components (or systems) must be characterized more rigorously than level A and B components in view of the extractables profile.
Additives: For level A components reference to 21 CFR Indirect Food Additive regulations is sufficient, for level B components additives are determined by testing, and for level C components extraction studies have to be performed.
After free registration in the Pharmarcopoeial Forum you can read the complete drafts of the new general chapter <661.3> and the revised chapter <1661>.
/////USP, draft, new general chapter, <661.3>, plastic components, manufacturing





















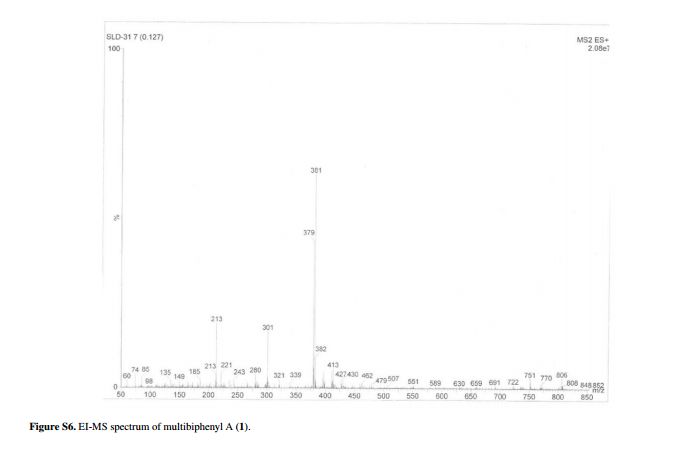
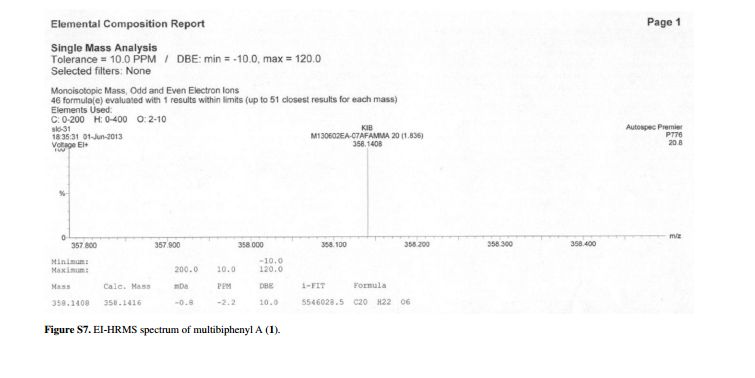





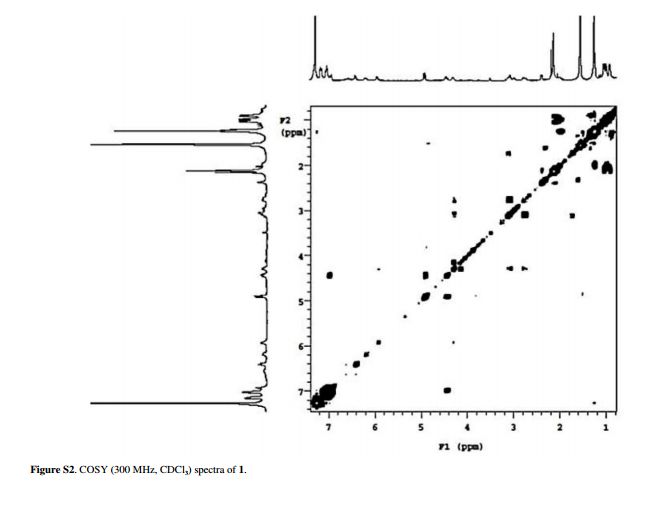

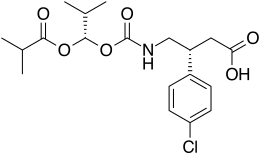










.bmp)
.bmp)
.gif)