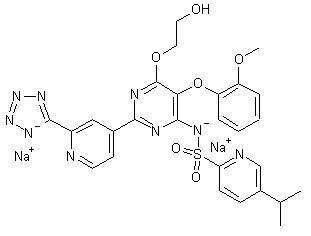
TEZOSENTAN
180384-57-0 CAS OF FREE ACID
N-[6-(2-Hydroxyethoxy)-5-(2-methoxyphenoxy)-2-[2-(2H-tetrazol-5-yl)pyridin-4-yl]pyrimidin-4-yl]-5-propan-2-ylpyridine-2-sulfonamide
5-isopropyl-pyridine-2-sulphonic acid 6-(2-hydroxy-ethoxy)-5- (2-methoxy-phenoxy)-2-(2-1 H-tetrazol-5-yl-pyridin-4-yl)- pyrimidin-4-ylamide
| Formula | C27H27N9O6S |
|---|---|
| Mol. mass | 605.624 |
…………………………………………………………………………….
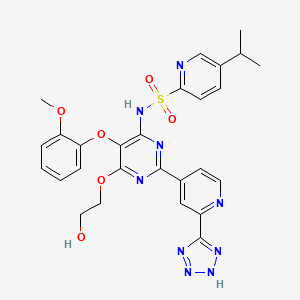
Tezosentan disodium, Ro-61-0612, Veletri
5-isopropyl-pyridine-2-sulfonic acid [6-(2-hydroxy-ethoxy)-5-(2-methoxy-phenoxy)-2-[2-(1H-tetrazole-5-yl)-pyridine-4-yl]-pyrimidine-4-yl]-amide sodium salt (1:2)
180384-58-1 of disodium salt, 180384-57-0 (free acid)
Tezosentan is a non-selective ETA and ETB receptor antagonist.[1] It acts as a vasodilator and was designed as a therapy for patients with acuteheart failure. Recent studies have shown however, that tezosentan does not improve dyspnea or reduce the risk of fatal or nonfatal cardiovascular events.[2]
Pulmonary disease (COPD), which may possibly be associated with pulmonary hypertension, as well as allergic and non-allergic rhinitis, provided that treatment with endothelin from a therapeutic standpoint is not contraindicated.
Tezosentan disodium is an endothelin ETB receptor antagonist in phase II clinical development for the treatment of stable, chronic pulmonary arterial hypertension. The drug was previously being evaluated for heart failure, but trials in that indication have been discontinued. The compound is being developed by Actelion.
………………………………..
SYNTHESIS

………………………………..
SYNTHESIS
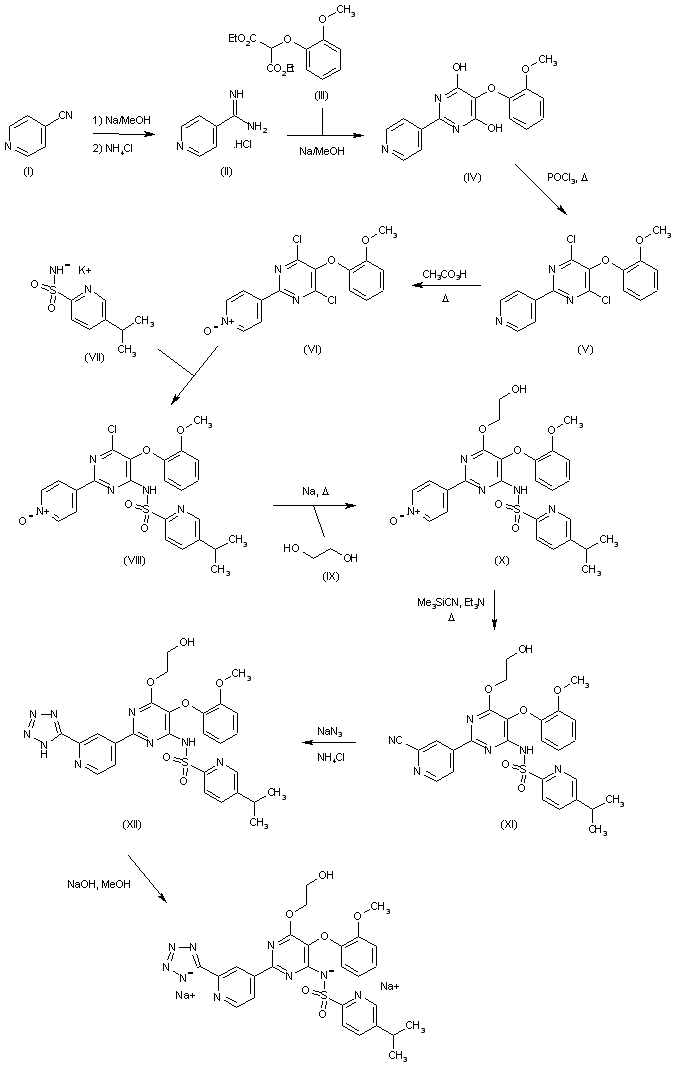
Reaction of 4-cyano-pyridine (I) with Na in methanol followed by treatment with ammonium chloride provides 4-amidino-pyridine hydrochloride (II), which is then converted into 5-(2-methoxyphenoxy)-2-(pyridin-4-yl)-pyrimidine-4,6-diol (IV) by condensation with diethyl malonate derivative (III) by means of Na in MeOH. By heating compound (IV) with phosphorus oxychloride (POCl3), 4,6-dichloro-5-(2-methoxyphenoxy)-2-pyridin-4-yl)pyrimidine (V) is obtained, which in turn is oxidized with peracetic acid in refluxing acetonitrile to afford N-oxide derivative (VI). Condensation of (VI) with 5-isopropylpyridine-2-sulfonamide potassium (VII) furnishes 5-isopropylpyridine-2-sulfonic acid 6-chloro-5-(2-methoxyphenoxy)-2-(1-oxy-pyridin-4-yl)-pyrimidin-4-yl amide (VIII), which is then dissolved in dimethoxyethane and subjected to reaction with Na in hot ethylene glycol (IX) to provide N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2-(1-oxy-pyridin-4-yl)-pyrimidin-4-yl]-5-isopropylpyridine-2-sulfonamide (X). Refluxing of (X) with trimethylsilylcyanide and Et3N in acetonitrile yields cyano derivative (XI), which is then converted into the tetrazole derivative (XII) by reaction with sodium azide and NH4Cl in DMF at 70 C. Finally, the disodium salt of tezosentan is obtained by treatment of (XII) with Na/MeOH in THF. refEP 0799209; JP 1998509182; WO 9619459
…………………………………..
SYNTHESIS PROCEDURE as in EP0979822A1
Examples
-
1360 ml of formamide were added to 136 g (437 mmol) of 5-(2-methoxy-phenoxy)-2-pyridine-4-yl-pyrimidine-4,6-diole. Then, at a temperature of 0°C, 11.7 ml (219 mmol) of concentrated sulfuric acid and thereafter 36.5 g (130 mmol) of iron(II)sulfate heptahydrate were added to the suspension. After that, 89 ml (874 mmol) of 30% hydrogen peroxide were added dropwise within 1 hr at a temperature of 0°C to 5°C. The viscous yellow-brownish suspension was stirred at 0°C for 1.5 hr. Subsequently, a solution of 83 g (437 mmol) of sodium pyrosulfite in 680 ml of de-ionized water was added dropwise to the reaction mixture within 30 min. at 0°C to 5°C and the reaction mixture was stirred at 0°C to 5°C for 30 min. The suspension was then filtered under reduced pressure. The filtrate was first washed with 1750 ml of de-ionized water and thereafter with 700 ml of ethanol. Then the solid was dried at 80°C, 2000 Pa for 16 hr. There were obtained 132.4 g (91% of theory) of 4-[4,6-dihydroxy-5-(2-methoxy-phenoxy)-pyrimidine-2-yl]-pyridine-2-carboxylic acid amide with a HPLC purity of 91.4% (w/w).
-
Preparation of starting material:
- a) 53.1 g of 4-cyano-pyridine (98%) are added all at once to a solution of 1.15 g of sodium in 200 ml of abs. MeOH. After 6 hr 29.5 g of NH4Cl are added while stirring vigorously. The mixture is stirred at room temperature overnight. 600 ml of ether are added thereto, whereupon the precipitate is filtered off under suction and thereafter dried at 50°C under reduced pressure. There is thus obtained 4-amidino-pyridine hydrochloride (decomposition point 245-247°C).
- b) 112.9 g of diethyl (2-methoxyphenoxy)malonate are added dropwise within 30 min. to a solution of 27.60 g of sodium in 400 ml of MeOH. Thereafter, 74.86 g of the amidine hydrochloride obtained in a) are added all at once. The mixture is stirred at room temperature overnight and evaporated at 50°C under reduced pressure. The residue is treated with 500 ml of ether and filtered off under suction. The filter cake is dissolved in 1000 ml of H2O and treated little by little with 50 ml of CH3COOH. The precipitate is filtered off under suction, washed with 400 ml of H2O and dried at 80°C under reduced pressure. There is thus obtained 5-(2-methoxy-phenoxy)-2-(pyridine-4-yl)-pyrimidine-4,6-diole (or tautomer), melting point above 250°C.
- Example 1
Example 2
-
Within 20 min. 61 ml (633 mmol) of POCl3 were added dropwise to 34 ml (200 mmol) of diisopropyl ethylamine at 5°C to 10°C followed by stirring at 5°C to 10°C for 15 min. Then 23.5 g (66 mmol) of 4-[4,6-dihydroxy-5-(2-methoxy-phenoxy)-pyrimidine-2-yl]-pyridine-2-carboxylic acid amide were added in four portions under cooling followed by stirring at 90°C for 25 hr. The reaction mixture was cooled down to 20°C and transferred to a new flask together with 50 ml of dichloromethane. Volatile components (i.e. excess of POCl3) was removed by evaporation from 20°C to 70°C followed by re-distillation with 100 ml of toluene. After adding 250 ml of dichloromethane to the residue (88 g of a black oil) the solution was heated to 35°C to 40°C and 80 ml of de-ionized water were added dropwise within 30 min. whereby the pH was kept constant by the subsequent addition of 28% NaOH solution (60 ml) within 5 to 6 hr. The mixture was stirred at 35°C to 40°C for 30 min. followed by removal of dichloromethane by distillation. The resulting suspension was allowed to cool down to 20°C and was stirred for additional 2 hr. The solid was filtered off under suction, washed with 500 ml of water and dried at 70°C, 2000 Pa for 16 hr. There were obtained 21.3 g (86% of theory) of 4-[4,6-dichloro-5-(2-methoxy-phenoxy)-pyrimidine-2-yl]-pyridine-2-carbonitrile with a HPLC purity of 94.3% (w/w).
-
8.95 g (24 mmol) of 4-[4,6-dichloro-5-(2-methoxy-phenoxy)-pyrimidine-2-yl]-pyridine-2-carbonitrile were suspended in 100 ml of acetone. At a temperature of 20°C, 5.04 g (25 mmol) of 5-isopropyl-pyridine-2-sulfonamide, 1 ml of de-ionized water, 10.6 g (77 mmol) of potassium carbonate and 135 mg (1.2 mmol) 1,4-diazobicyclo[2.2.2]octane were added. The mixture was stirred at 40°C for 20 hr. Thereafter, another 240 mg (1.2 mmol) of 5-isopropyl-pyridine-2-sulfonamide and 80 mg (0.7 mmol) of 1,4-diazobicyclo[2.2.2]octane were added. The reaction mixture was stirred for 24 hr at 40°C followed by cooling to 20°C. Then 50 ml of de-ionized water and 45 ml of 3 N aqueous hydrochloric acid were added slowly until pH = 1. The acetone was removed by distillation and the resulting suspension was stirred at 20°C for 1.5 hr. The solid was filtered off under suction, washed first with 100 ml of de-ionized water and thereafter with 50 ml of t-butylmethylether. Then the solid was dried at 70°C, 2000 Pa for 20 hr. There were obtained 13.2 g (102% of theory) of 5-isopropyl-pyridine-2-sulfonic acid [6-chloro-2-(2-cyano-pyridine-4-yl)-5-(2-methoxy-phenoxy)-pyrimidine-4-yl]-amide with a HPLC purity of 87.8% (w/w).
- Example 4
-
122 g (233 mmol) of 5-isopropyl-pyridine-2-sulfonic acid [6-chloro-2-(2-cyano-pyridine-4-yl)-5-(2-methoxy-phenoxy)-pyrimidine-4-yl]-amide was suspended in 450 ml of N,N-dimethyl formamide and the mixture was cooled down to 15°C. At this temperature, 35 ml of hydrazine hydrate were added dropwise within 1 hr. The resulting solution was stirred at 15°C to 20°C for 16 hr and thereafter diluted with 600 ml of de-ionized water. Then 50 ml of glacial acetic acid were added dropwise at 0°C to 5°C until pH = 5.5. 600 g of ice were added and the suspension was stirred for 1 hr. The solid was filtered off under suction, washed with 3000 ml of water and dried at 40°C, 2000 Pa for 24 hr. There were obtained 126 g (97% of theory) of 5-isopropyl-pyridine-2-sulfonic acid [6-chloro-2-[2-(hydrazino-imino-methyl)-pyridine-4-yl]-5-(2-methoxy-phenoxy)-pyrimidine-4-yl]-amide with a HPLC purity of 91.8% (w/w).
- Example 6
-
20 g (35 mmol) of 5-isopropyl-pyridine-2-sulfonic acid [6-chloro-2-[2-(hydrazino-imino-methyl)-pyridine-4-yl]-5-(2-methoxy-phenoxy)-pyrimidine-4-yl]-amide were added to 160 ml of N,N-dimethyl formamide. The solution was kept at 15°C to 20°C and 23 ml of 6 N aqueous hydrochloric acid were added, followed by addition of a solution containing 4.8 g (7 mmol) of sodium nitrite in 20 ml de-ionized water within 10 min. The mixture was stirred at 20°C for 1 hr, then 140 ml of de-ionized water were added and the suspension was stirred at 0°C for 1 hr. The solid was filtered, firstly washed with 80 ml of de-ionized water and thereafter with 80 ml of t-butylmethylether. Then the solid was dried at 70°C and 2000 Pa for 16 hr. The crude product (23.4 g) was taken up with 117 ml of tetrahydrofuran for 1 hr. After filtration at 0°C the crystallized product was washed with 25 ml of t-butylmethylether and was then dried at 70°C, 2000 Pa for 16 hr. There were obtained 17.3 g (84% of theory) of 5-isopropyl-pyridine-2-sulfonic acid [6-chloro-5-(2-methoxy-phenoxy)-2-[2-(1H-tetrazole-5-yl)-pyridine-4-yl]-pyrimidine-4-yl]-amide with a HPLC purity of 91.1% (w/w).
- Example 8
- Example 10
-
6.2 g of sodium hydroxide were added to 15 g (26 mmol) of 5-isopropyl-pyridine-2-sulfonic acid [6-chloro-5-(2-methoxy-phenoxy)-2-[2-(1H-tetrazole-5-yl)-pyridine-4-yl]-pyrimidine-4-yl]-amid and 75 ml of ethylene glycol. The mixture was heated to 85°C for 5 hr. Then 55 ml of de-ionized water were added and thereafter 55 ml of 3 N hydrochloric acid were added dropwise. The mixture was allowed to cool down to 20°C and was stirred for 1 hr. The solid was filtered off and dried at 70°C, 2000 Pa for 18 hr. There were obtained 16.2 g (103%) of 5-isopropyl-pyridine-2-sulfonic acid 16-(2-hydroxy-ethoxy)-5-(2-methoxy-phenoxy)-2-[2-(1H-tetrazole-5-yl)-pyridine-4-yl]-pyrimidine-4-yl]-amide with a HPLC purity of 92% (w/w). 80 ml of dioxane and 80 ml of ethanol were added to this solid. At a temperature of 60°C, gaseous ammonia was introduced into the liquid until pH = 9 to 10. The resulting suspension was allowed to cool down to 20°C and was stirred at 20°C for 20 hr and thereafter at 0°C for 2.5 hr. Then the solid was filtered off and dried at 70°C, 2000 Pa for 18 hr. There were obtained 14.2 g of mono ammonium salt with a HPLC purity of 96.2% (w/w). The solid was heated (reflux) in 70 ml of methanol, cooled down slowly to 20°C and stirred at 20°C for 19 hr and thereafter at 0°C for 2 hr. Then the solid was filtered off and dried at 70°C, 2000 Pa for 19 hr. There were obtained 11.5 g (66% of theory) of 5-isopropyl-pyridine-2-sulfonic acid [6-(2-hydroxy-ethoxy)-5-(2-methoxy-phenoxy)-2-[2-(1H-tetrazole-5-yl)-pyridine-4-yl]-pyrimidine-4-yl]-amide sodium salt (1:2) with a HPLC purity of 98.6% (w/w).
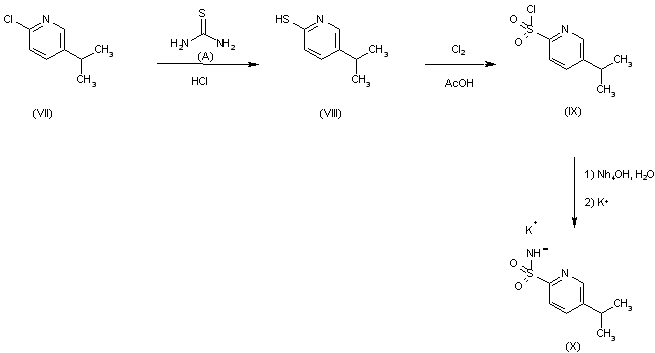
Reaction of 2-chloro-5-ispropylpyridine (VII) with thiourea (A) in aqueous HCl gives 5-isopropyl- pyridine-2-thiol (VIII), which is chlorinated with chlorine in acetic acid to yield 5-isopropylpyridine-2-sulfochloride (IX). This compound is converted into 5-isopropylpyridine-2-sulfonamide potassium salt (X).
…………………………
synthesis
. Example 1
a) 200 ml of dimethoxyethane and 1 10.9 g of 4-[4-(4-tert- butyl-phenyl-sulphonylamino)-6-chloro-5-(2-methoxy-phenoxy)- pyrimidin-2-yl]-pyridine 1 -oxide are added all at once to a solution of 23.80 g of sodium in 660 ml of ethylene glycol. The solution is heated at 90°C for 20 hours while stirring, thereafter cooled, poured into 2500 ml of H2O and thereafter treated with CH3COOH to pH 5. The mixture is extracted three times with EtOAc, the organic phase is washed with H2O, dried with Na2Sθ4 and evaporated under reduced pressure. The residue is recrystall- ized from CH3CN and thereafter twice from a mixture of acetone and CH3CN. There is thus obtained 4-[4-(4-tert-butyl-phenyl- sulphonylamino)-6-(2-hydroxy-ethoxy)-5-(2-methoxy-phenoxy)- pyrimidin-2-yl]-pyridine 1 -oxide.
Preparation of the starting material:
b) 53.1 g of 4-cyano-pyridine (98%) are added all at once to a solution of 1.15 g of sodium in 200 ml of abs. MeOH. After
6 hours 29.5 g of NH4CI are added while stirring vigorously. The mixture is stirred at room temperature overnight. 600 ml of ether are added thereto, whereupon the precipitate is filtered off under suction and thereafter dried at 50°C under reduced pressure. There is thus obtained 4-amidino-pyridine hydro- chloride (decomposition point 245-247°C).
c) 1 12.9 g of diethyl (2-methoxyphenoxy)malonate are added dropwise within 30 minutes to a solution of 27.60 g of sodium in 400 ml of MeOH. Thereafter, 74.86 g of the amidine hydro- chloride obtained in b) are added all at once. The mixture is stirred at room temperature overnight and evaporated at 50°C under reduced pressure. The residue is treated with 500 ml of ether and filtered off under suction. The filter cake is dissolved in 1000 ml of H2O and treated little by little with 50 ml of CH3COOH. The precipitate is filtered off under suction, washed with 400 ml of H2O and dried at 80°C under reduced pressure. There is thus obtained 5-(2-methoxy-phenoxy)-2-(pyridin-4-yl)- pyrimidine-4,6-diol (or tautomer), melting point above 250°C.
d) A suspension of 1 54.6 g of 5-(2-methoxy-phenoxy)-2- (pyridin-4-yl)-pyrimidine-4,6-diol (or tautomer) in 280 ml of POCI3 is heated at 120°C in an oil bath for 24 hours while stirring vigorously. The reaction mixture changes gradually into a dark brown liquid which is evaporated under reduced pressure and thereafter taken up three times with 500 ml of toluene and evaporated. The residue is dissolved in 1000 ml of CH2CI2, treated with ice and H2O and thereafter adjusted with 3N NaOH until the aqueous phase has pH 8. The organic phase is separated and the aqueous phase is extracted twice with CH2CI2. The combined CH2CI2 extracts are dried with MgSθ4, evaporated to half of the volume, treated with 1000 ml of acetone and the CH2CI2 remaining is distilled off at normal pressure. After standing in a refrigerator for 2 hours the crystals are filtered off under suction and dried at 50°C overnight. There is thus obtained 4,6-dichloro-5-(2-methoxy-phenoxy)-2-pyridin-4-yl)- pyrimidine, melting point 1 78-1 80°C.
e) A solution of 1 7.4 g of 4,6-dichloro-5-(2-methoxy- phenoxy)-2-pyridin-4-yl)-pyrimidine in 100 ml of CH3CN is boiled at reflux for 3 hours with 1 5 ml of a 32% peracetic acid solution, thereafter cooled and stored in a refrigerator overnight. The crystals are filtered off under suction and dried at 50°C under reduced pressure. There is thus obtained 4-[4,6-dichloro- 5-(2-methoxy-phenoxy)-pyrimidin-2-yl]-pyridine 1 -oxide, melting point 189-1 90°C.
f) A solution of 36.4 g of 4-[4,6-dichloro-5-(2-methoxy- phenoxy)-pyrimidin-2-yl]-pyridine 1 -oxide and 52.8 g of p-tert- butylphenyl-sulphonamide potassium in 1 50 ml of abs. DMF is stirred at room temperature for 24 hours. Thereafter, it is poured into a mixture of 1 500 ml of H2O and 1000 ml of ether while stirring mechanically, whereby a precipitate forms. The suspension is adjusted to pH 5 with CH3COOH, suction filtered, the crystals are washed with cold water and thereafter with ether and dried at 50°C. There is thus obtained 4-[4-(4-tert- butyl-phenylsulphonylamino)-6-chloro-5-(2-methoxy-phenoxy)- pyrimidin-2-yl]-pyridine 1 -oxide as a colourless material of melting point 247-249°C.
Example 2
A solution of 78.45 g of 4-[4-(4-tert-butyl-phenyl- sulphonylamino)-6-(2-hydroxy-ethoxy)-5-(2-methoxy-phenoxy)- pyrimidin-2-yl]-pyridine 1 -oxide, 122.5 g of trimethylsilyl cyanide, 127.8 g of triethylamine and 1200 ml of CH3CN is boiled at reflux for 20 hours and thereafter evaporated under reduced pressure. The oily residue is taken up in 1000 ml of EtOAc and the solution is washed with CH3COOH:H2θ 9:1 and then with H2O. The EtOAc extracts are dried with Na2SO4. After evaporation of the solvent the residue is taken up in a mixture of CH3CN and CF3COOH (20:1 ), whereby a crystalline precipitate separates. There is thus obtained 4-tert-butyl-N-[2-(2-cyano-pyridin-4- yl)-6-(2-hydroxy-ethoxy)-5-(2-methoxy-phenoxy)-pyrimidin-4- yl]-benzenesulphonamide of melting point 176-1 79°C.
Example 3 for analogy only compd is different
A suspension of 50.0 g of 4-tert-butyl-N-[2-(2-cyano- pyridin-4-yl)-6-(2-hydroxy-ethoxy)-5-(2-methoxy-phenoxy)- pyrimidin-4-yl]-benzenesulphonamide, 46.33 g of NH4CI and 56.47 g of NaN3 in 1600 ml of DMF is heated to 70°C for 24 hours while stirring vigorously. The majority of the solvent is distilled off under reduced pressure, the residue is dissolved in H2O, the solution is extracted four times at pH 6.5 with ether, thereafter treated with CH3COOH to pH = 4.5 and extracted with EtOAc. After working up there is obtained a residue which is treated with ether and filtered off under suction therefrom. There is thus obtained 4-tert-butyl-N-[6-(2-hydroxy-ethoxy)-5-(2- methoxy-phenoxy)-2-(2-1 H-tetrazol-5-yl-pyridin-4-yl)- pyrimidin-4-yl]-benzenesulphonamide, melting point 225-227°C.
Example 30 final product
In analogy to Example 3, from 5-isopropyl-pyridine-2- sulphonic acid 2-(2-cyano-pyridin-4-yl)-6-(2-hydroxy-ethoxy)- 5-(2-methoxy-phenoxy)-pyrimidin-4-ylamide there is obtained 5-isopropyl-pyridine-2-sulphonic acid 6-(2-hydroxy-ethoxy)-5- (2-methoxy-phenoxy)-2-(2-1 H-tetrazol-5-yl-pyridin-4-yl)- pyrimidin-4-ylamide (tezosantan free base) as a white substance of melting point 1 98- 200°C from acetonitrile.
The corresponding disodium salt (tezosantan di sodium salt) is obtained as a white powder from this product using sodium methylate in analogy to Example 5
Example 5 for analogy only, compd is different
A solution of 47.8 g of 2-[6-(4-tert-butyl-phenylsulphonyl- amino)-5-(2-methoxy-phenoxy)-2-(2-1 H-tetrazol-5-yl-pyridin- 4-yl)-pyrimidin-4-yloxy]-ethyl pyridin-2-ylcarbamate in 500 ml of abs. THF is treated dropwise with a cold solution of 2.8 g of sodium in 50 ml of methanol, whereby there forms gradually a solid precipitate which, after stirring at room temperature for 1 hour, is filtered off under suction, dried under greatly reduced pressure at 35°C for 3 days and thereafter at 50°C for 2 days. There is thus obtained the bis-sodium salt, decomposition point above 250°C.
References
- Urbanowicz, W; Sogni, P, Moreau, R, Tazi, K A, Barriere, E, Poirel, O, Martin, A, Guimont, M C, Cazals-Hatem, D, Lebrec, D (2004). “Tezosentan, an endothelin receptor antagonist, limits liver injury in endotoxin challenged cirrhotic rats”. Gut (BMJ Publishing Group Ltd & British Society of Gastroenterology) 53 (12): 1844–1849. doi:10.1136/gut.2003.036517. PMC 1774327. PMID 15542526.
- “Tezosentan does not appear to improve symptoms for patients with acute heart failure”. Medical Studies/Trials. news-medical.net. 7 Nov 2007. Retrieved 2007-11-24.
4 US2003/100507 A1
5 Drugs Fut 2003,28(8),754
6 WO 1996019459……
7 EP 0897914
8 WO 2011163085
9 WO 2004082637
| 10 WO 2002074034 |
11…
|
4-8-2004
|
Discovery, modeling, and human pharmacokinetics of N-(2-acetyl-4,6-dimethylphenyl)-3-(3,4-dimethylisoxazol-5-ylsulfamoyl)thiophene-2-carboxamide (TBC3711), a second generation, ETA selective, and orally bioavailable endothelin antagonist.
|
Journal of medicinal chemistry
|
12 ..
|
7-1-1999
|
RO 610612 .
|
Drugs in R&D
|
13….














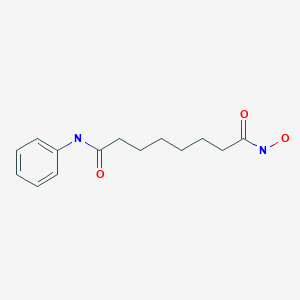 VORINOSTAT
VORINOSTAT






















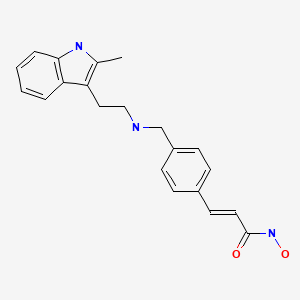 panobinostat
panobinostat












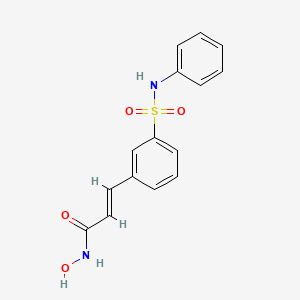 BELINOSTAT
BELINOSTAT





















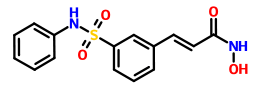

















 CLAZOSENTAN
CLAZOSENTAN CLAZOSENTAN DI-NA SALT is discontinued
CLAZOSENTAN DI-NA SALT is discontinued