Engineering of a fungal laccase to develop a robust, versatile and highly-expressed biocatalyst for sustainable chemistry
Abstract
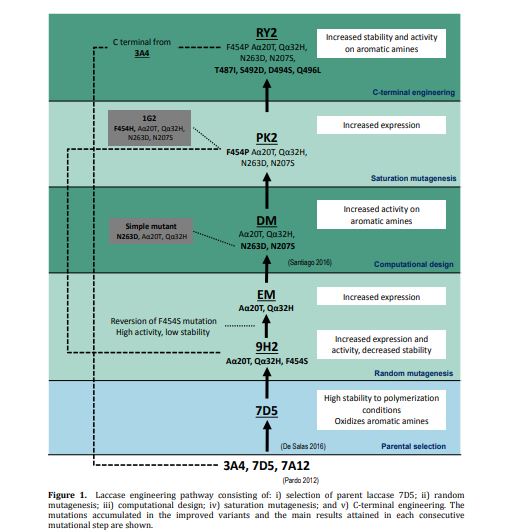
Fungal laccases can play an important role as biocatalysts in organic chemistry to replace chemical synthesis. In a previous work we synthesized conductive polyaniline using a high-redox potential laccase from our collection of recombinant fungal variants. Still, the oxidation of aniline is hindered by the reaction conditions (low pH and presence of anionic surfactants). Thus, we tackle here the directed evolution of the enzyme asisted by computational simulation aiming at improving aniline oxidation at the required polymerization conditions while maintaining the enzyme´s substrate promiscuity. Simultaneously, its secretion by the host used for the engineering (Saccharomyces cerevisiae) was enhanced. Then, the improved laccase variant was overproduced in the industrial host Aspergillus oryzae and assayed for one‐pot synthesis of polyaniline and naphtol-derived dyes whose textile dyeing properties were verified in an industrial environment. Finally, modification of its C-terminal tail further enhanced laccase stability by flexibilization of the region. The resulting biocatalyst displays noticeable stability at high temperature and extreme pH while shows improved kcat values on the different substrates tested. Moreover, it is remarkably produced in S. cerevisiae at rates not formerly reported in the literature. These facts, together with the overexpression in A. oryzae opens new scenarios for its further development and application.
Enzyme production and purification Laccase was produced by S. cerevisiae 1 L-flask cultures and purified as described before.5 Laccase activity in the culture was measured spectrophotometrically with 3 mM ABTS in 100 mM citrate-phosphate buffer, pH 3 by the increase of Absorbance 418 nm (ε418 = 36000 M−1 cm−1). One activity unit (U) was defined as the amount of enzyme needed to transform 1 µmol substrate/minute. To estimate enzyme production, enzyme concentration of a purified laccase variant was measured by the A280 (Nanodrop 2000, Thermofisher, USA) and the specific activity (U/mg) was calculated and used to deduce the mg of enzyme/l obtained in the culture. Enzyme characterization All characterization assays were performed with purified enzymes.
////////
https://pubs.rsc.org/en/content/articlepdf/2019/gc/c9gc02475a













Sorry, the comment form is closed at this time.