Recent progress on fluorination in aqueous media
DOI: 10.1039/C7GC01566F, Tutorial Review
Advances of fluorination in aqueous media during the last few decades are summarized in this review
Recent progress on fluorination in aqueous media
*Corresponding authors
Abstract
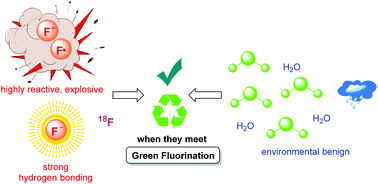
Advances in aqueous fluorination during the last few decades are summarized in this review. Fluorinated compounds have dominated a large percentage of agrochemicals and pharmaceuticals and a mass of functional materials. The incorporation of fluorine atoms into organic molecules has become one of the mainstream technologies for their functional modification. Water is very environmentally friendly and has advantageous physicochemical properties. Fluorination reactions in aqueous media are highly sought-after, and have attracted great attention in research areas ranging from medicinal chemistry to materials science. In early times and for a long time, fluorination was thought to be diametrically opposed to water or moisture. However, recent examples have conflicted with this viewpoint. The successful merger of “untamed” fluorine and “mild” water in chemical reactions has set up a new prospect for green chemistry. A considerable amount of remarkable research works have been carried out using water as a (co)solvent and/or a reactant for transformations including electrophilic, radical, or nucleophilic fluorination. We hope that this review will serve as a guide to better understand and to further broaden the field of fluorine chemistry in aqueous conditions.
Conclusion
The installation of fluorine atoms into organic and organometallic frameworks can dramatically change their physical, chemical, and biological properties. Organofluorides have entered many fields of science and technology with a tremendous impact on these domains. The development of efficient, selective, and mild methods to build C-F bonds is of great importance, which is highly desirable to keep up with the rapidly growing demand of novel fluorine-containing scaffolds. In early times, most fluorination reactions required harsh conditions and moisture-sensitive, highly toxic, and explosive atomic fluorine transfer agents like fluorine gas, xenon difluoride, hypofluorite, antimonytrifluoride, and diethylaminosulfurtrifluoride. The discovery of stable electrophilic fluorination reagents such as Selectflour and NFSI has remarkably changed the dilemma, which realized a large number of safe, mild, and easily controllable electrophilic and radical fluorination reactions in aqueous media. Although the exact mechanisms are still unclear at present, it does never hamper the green fluorination method development with these reagents. A mass of successful examples have confirmed that the aqueous reaction medias have positive impacts on electrophilic and radical fluorination reactions with using the N-F reagents and in many cases water can also be a nucleophile for the entire conversions.
In addition, water was generally thought to be an unsuitable medium for nucleophilic fluorination because the fluoride ions can be “trapped” in aqueous medias by hydrogen bonding and become unreactive. Thus, their use in organic synthesis has been quite limited to polar aprotic solvents. Although the strong hydrogen bond formed between fluoride and water diminished the nucleophilicity of fluoride ions, the recent examples of nucleophilic fluorination in aqueous media have implied that this “negative” effect does not always harm the reaction. Besides, the radioisotope 18F has been considered to be a good choice for PET imaging owing to its desirable radiochemical properties. With a half-life of 110 minutes, the introduction of [ 18F]fluorine atoms into biomolecules has to be completed in a swift manner to minimize the loss of radioactivity. Nucleophilic incorporation of [18F]F‒ in aqueous conditions could rapidly produce [18F]fluorinesubstituted biomolecules, which avoided azeotropic drying process, shortened the production time, and minimized the loss of activity. We summarized the recent aqueous fluorination reactions in three sections according to their possible mechanisms. The successful amalgamation of “ill-tempered” fluorine and “benign” water has boded well for green fluorine chemistry. Water behaves as a cosolvent to dissolve fluorination reagents and/or as a reactant for bifunctionalization. Since the aspects of green chemistry has drawn much attention from the society, the pursuit of more efficient and milder reaction conditions for greener fluorination in aqueous medias will never end. Although a large number of research works have been published in this area, it’s only the tip of the iceberg with a wide scope for improvement. We hope that this review will serve as a guide to understand and to further broaden the field of aqueous fluorine chemistry. To meet the principle of green chemistry in modern synthesis, the development of new fluorination reagents as well as valid catalytic systems is crucial for mild and selective C-F bond formation. It’s undoubted that a growing number of green fluorination methodologies in aqueous media will be witnessed in the near future.
///////////













Sorry, the comment form is closed at this time.