Ecocatalyzed Suzuki cross coupling of heteroaryl compounds
DOI: 10.1039/C7GC01672G, Paper
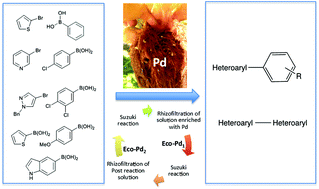
A bio-based EcoPd was developed for the Suzuki cross coupling of heteroaryl compounds.
Ecocatalyzed Suzuki cross coupling of heteroaryl compounds
Abstract
A bio-based EcoPd was developed for the Suzuki cross coupling of heteroaryl compounds. Based on the ability of Eichhornia crassipes to bioconcentrate Pd in its roots, we addressed the transformation of plant-derived Pd metals to green catalysts. The methodology is based on eco-friendly procedures. It allowed the preparation of a wide range of heterocyclic biaryl and heterocyclic–heterocyclic biaryl compounds, with a low Pd catalyst loading. EcoPd was found to have the ideal microstructure to promote complex Suzuki reactions without ligands or additives. For the first time, post-reaction solution was treated by rhizofiltration. The resulting EcoPd has been reused with the same performance. This work has established the ecocatalysis concept as a powerful strategy for Pd sustainability, with the development of homogeneous catalysts that are easily recycled and reused.
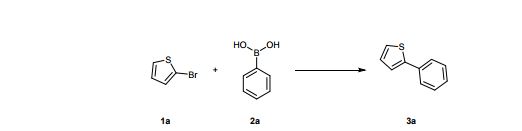
2-Bromothiophene (20 g, 125 mmol), Phenyl boronic acid (16.8 g, 138 mmol), potassium carbonate (20.7 g, 150 mmol) and EcoPd1 (113 mg, 125 µmol of Pd, 13.3 mg of Pd, EcoPd1 at 11.7 wt% of Pd) were suspended into degassed glycerol (200 mL). The mixture was stirred at 120°C for 4h thanks to an oil bath under an argon atmosphere. The reaction was checked for completion by TLC (cyclohexane) and GCMS analysis after a short extraction of the organic material: 10 µL of the crude were added into a 1 mL microtube containing a mixture of water and AcOEt (800 µL, 1:1, v/v) ; the microtube was vortexed before using the organic layer to perform analysis. Deionised water (500 mL) and AcOEt (500 mL) were added into the flask and the mixture filtered through fritted glass to isolate black Pd for recycling. The organic layer was further washed by deionised water (500 mL x 3) before drying over Na2SO4. The organic layer was filtered and concentrated under vacuum. The residue was then purified by chromatography on a silica gel column (250 g) with pure cyclohexane as the mobile phase, giving the desired coupled compound as a white powder (18 g, 112.5 mmol, yield 90%) Rf = 0.7 (cyclohexane).
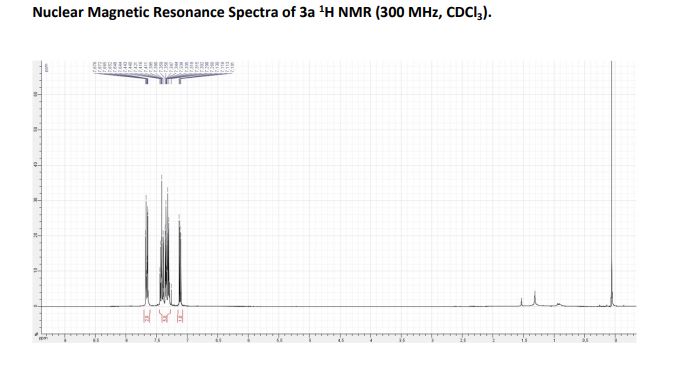
1H NMR (300 MHz, CDCl3): = 7.10- 7.13 (m, 2H), 7.44-7.26 (m, 5H), 7.38-7.33 (m, 1H).
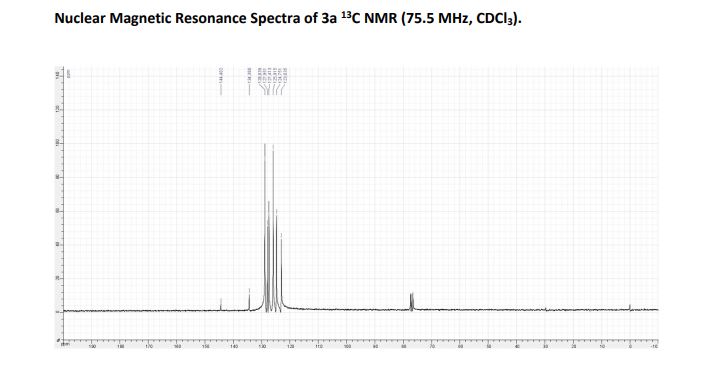
13C NMR (75.5 MHz, CDCl3): = 123.0, 124.8, 125.9, 127.4, 128.0, 128.8, 134.4, 144.4.
MS (EI): m/z = 160 (M+ , 100%), 128 (21%), 115 (54%), 89 (17%) calcd for C10H8S: 159.99.
////////













Sorry, the comment form is closed at this time.