Control of stereoselectivity of benzylic hydroxylation catalysed by wild-type cytochrome P450BM3 using decoy molecules
DOI: 10.1039/C7CY01130J, Paper
The benzylic hydroxylation of non-native substrates was catalysed by cytochrome P450BM3, wherein “decoy molecules” controlled the stereoselectivity of the reactions.
-
Catalysis Science & Technology
Control of stereoselectivity of benzylic hydroxylation catalysed by wild-type cytochrome P450BM3 using decoy molecules
Abstract
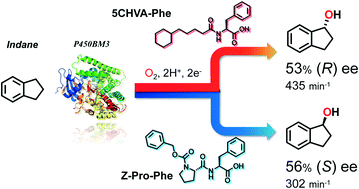
The hydroxylation of non-native substrates catalysed by wild-type P450BM3 is reported, wherein “decoy molecules”, i.e., native substrate mimics, controlled the stereoselectivity of hydroxylation reactions. We employed decoy molecules with diverse structures, resulting in either a significant improvement in enantioselectivity or clear inversion of stereoselectivity in the benzylic hydroxylation of alkylbenzenes and cycloalkylbenzenes. For example, supplementation of wild-type P450BM3 with 5-cyclohexylvaleric acid-L-phenylalanine (5CHVA-Phe) and Z-proline-L-phenylalanine yielded 53% (R) ee and 56% (S) ee for indane hydroxylation, respectively, although 16% (S) ee was still observed in the absence of any additives. Moreover, we performed a successful crystal structure analysis of 5CHVA-L-tryptophan-bound P450BM3 at 2.00 Å, which suggests that the changes in selectivity observed were caused by conformational changes in the enzyme induced by binding of the decoy molecules.

| M2 | Kazuto Suzuki | \ | suzuki.kazuto*c.mbox.nagoya-u.ac.jp |

| Yoshihito Watanabe | yoshi*nucc.cc.nagoya-u.ac.jp | ||
/////////////////














Sorry, the comment form is closed at this time.