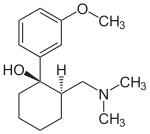
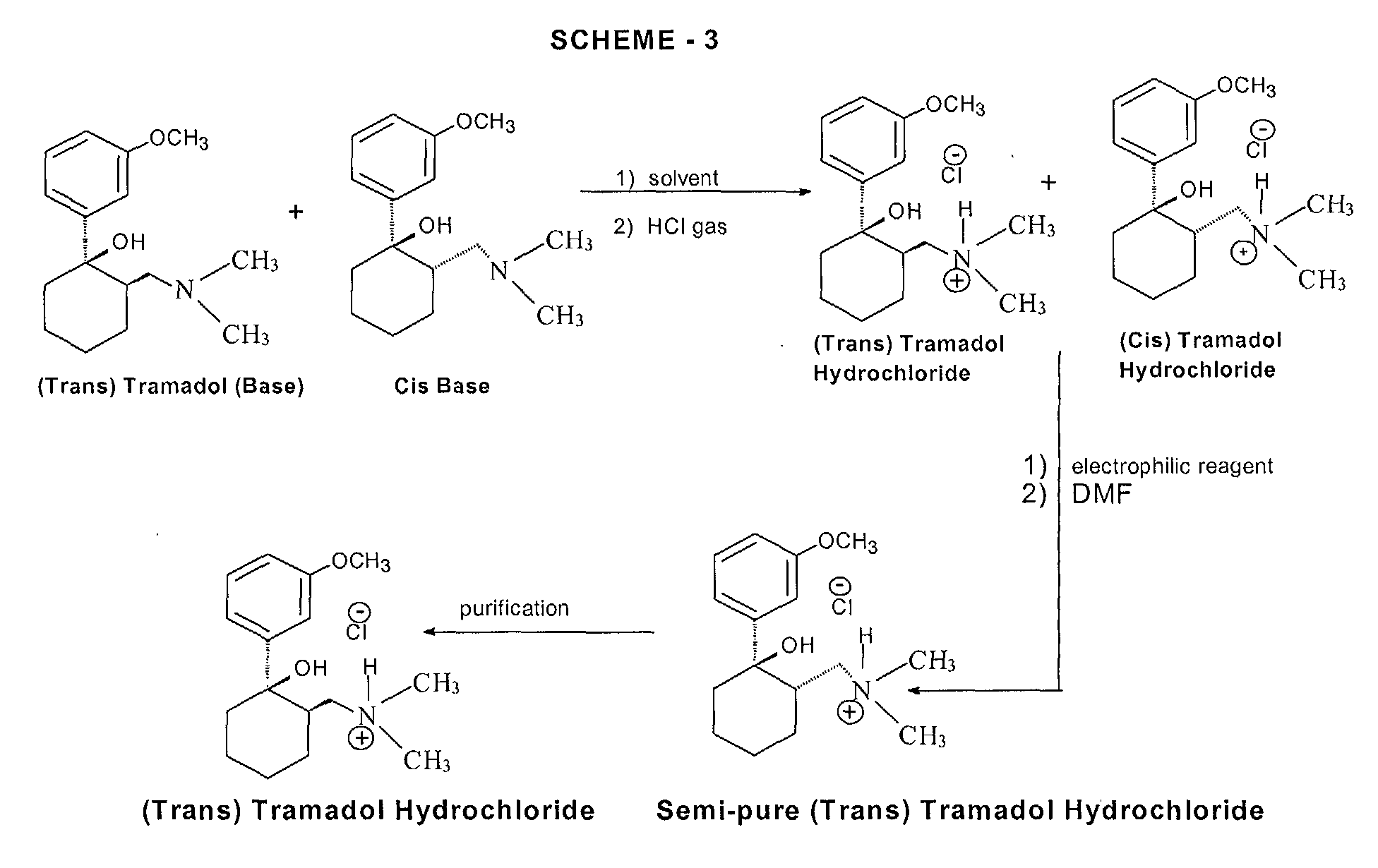
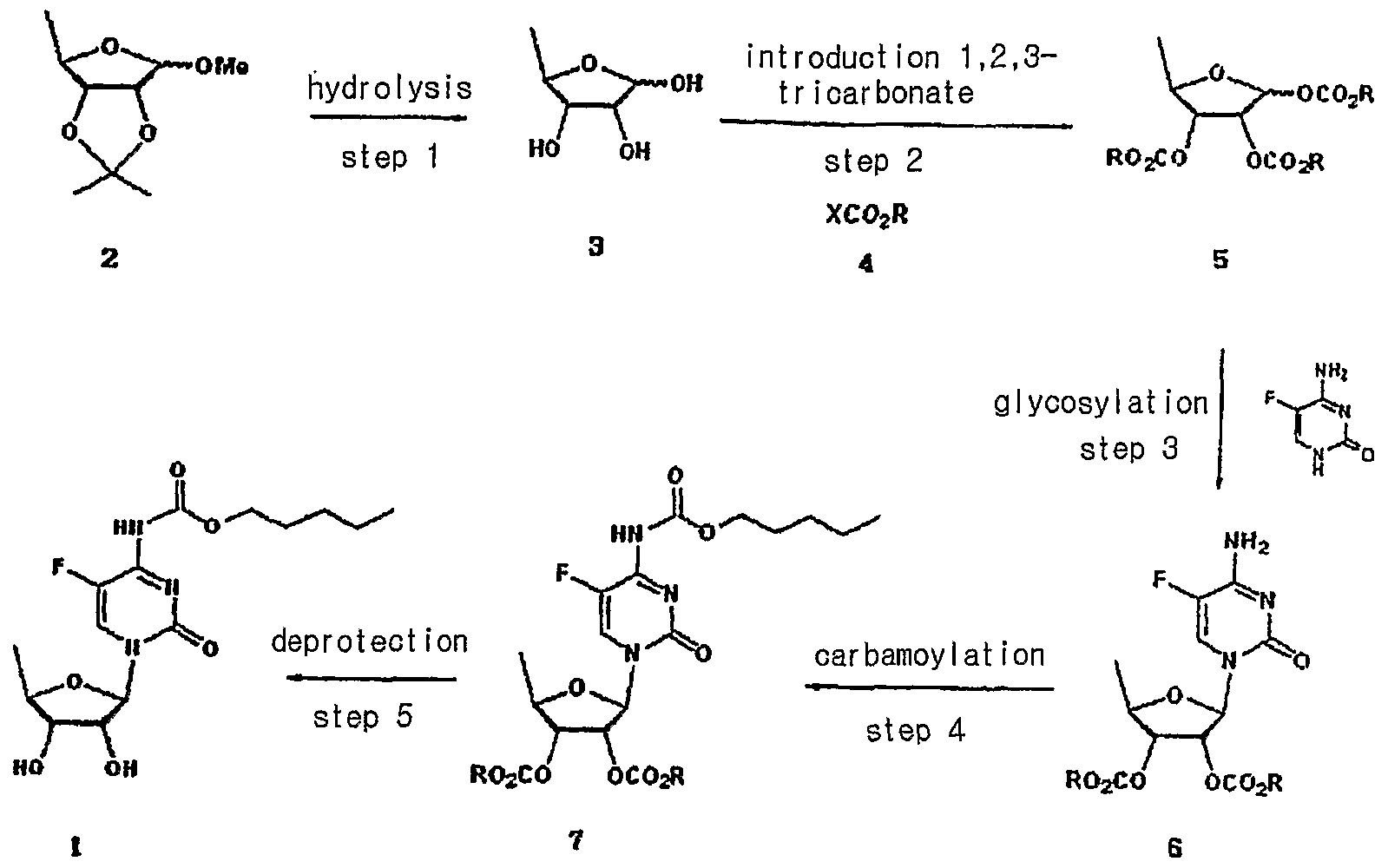
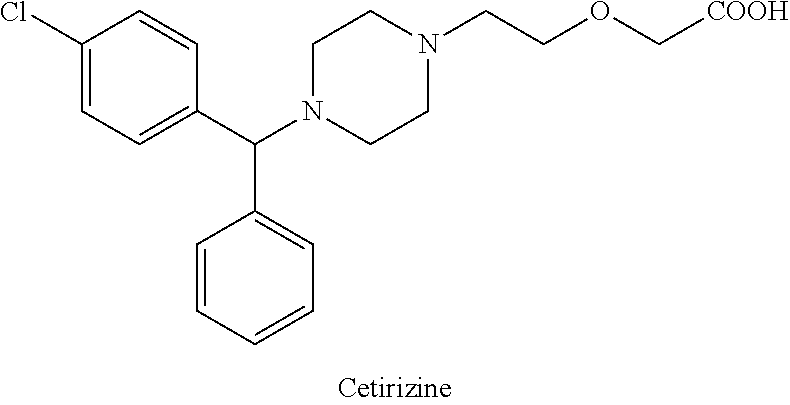
niaspan
Teva Announces Exclusive Launch of Generic NIASPAN® in the United States
JERUSALEM–(BUSINESS WIRE)–Teva Pharmaceutical Industries Ltd. (NYSE:TEVA) today announced the launch of the generic equivalent to NIASPAN® (niacin extended-release) tablets, 500, 700, and 1000mg in the United States. Teva was first to file, making the product eligible for 180 days of marketing exclusivity.
NIASPAN® is marketed by AbbVie and used with diet to reduce elevated TC, LDL-C, Apo B and TG levels, and to increase HDL-C in patients with primary hyperlipidemia and mixed dyslipidemia. NIASPAN® had annual sales of approximately $1.12 billion in the United States, according to IMS data as of June 30, 2013. read more at
http://www.pharmalive.com/teva-launches-generic-niaspan-in-us


niacin, vit B3
Niacin (also known as vitamin B3, nicotinic acid, or less commonly vitamin PP; archaic terms include pellagra-preventive and anti-dermatitis factor) is an organic compound with the formula C
6H
5NO
2 and, depending on the definition used, one of the 40 to 80 essential human nutrients.
Niacin is one of five vitamins (when lacking in human diet) associated with a pandemic deficiency disease: niacin deficiency (pellagra), vitamin C deficiency (scurvy), thiamin deficiency (beriberi), vitamin D deficiency (rickets and osteomalacia), vitamin A deficiency (night blindness and other symptoms). Niacin has been used for over 50 years to increase levels of HDL in the blood and has been found to decrease the risk of cardiovascular events modestly in a number of controlled human trials.[3]
This colorless, water-soluble solid is a derivative of pyridine, with a carboxyl group (COOH) at the 3-position. Other forms of vitamin B3 include the corresponding amide, nicotinamide (“niacinamide”), where the carboxyl group has been replaced by a carboxamide group (CONH
2), as well as more complex amides and a variety of esters. Nicotinic acid and niacinamide are convertible to each other with steady world demand rising from 8500 tonnes per year in 1980s to 40,000 in recent years.[4]
Niacin cannot be directly converted to nicotinamide, but both compounds could be converted to and are precursors of NAD andNADP in vivo.[5] Nicotinic acid, nicotinamide, and tryptophan (via quinoline acid) are co-factors for nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). NAD converts to NADP by phosphorylation in the presence of the enzyme NAD+ kinase. NADP and NAD are coenzyme for many dehydrogenases, participating in many hydrogen transfer processes.[6] NAD is important in catabolism of fat, carbohydrate, protein, and alcohol, as well as cell signaling and DNA repair, and NADP mostly in anabolism reactions such as fatty acid and cholesterol synthesis.[6] High energy requirements (brain) or high turnover rate (gut, skin) organs are usually the most susceptible to their deficiency.[7]
Although the two are identical in their vitamin activity, nicotinamide does not have the same pharmacological effects (lipid modifying effects) as niacin. Nicotinamide does not reduce cholesterol or cause flushing.[8] Nicotinamide may be toxic to the liver at doses exceeding 3 g/day for adults.[9] Niacin is involved in both DNA repair, and the production of steroid hormones in theadrenal gland.
- “Niacin”. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Retrieved 14-January-2012.
- PubChem 938
- Bruckert, E; Labreuche, J; Amarenco, P (2010 Jun). “Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis”.Atherosclerosis 210 (2): 353–61. doi:10.1016/j.atherosclerosis.2009.12.023.PMID 20079494.
- Cantarella, L; Gallifuoco, A; Malandra, A; Martínková, L; Spera, A; Cantarella, M (2011). “High-yield continuous production of nicotinic acid via nitrile hydratase-amidase cascade reactions using cascade CSMRs”. Enzyme and microbial technology 48 (4–5): 345–50. doi:10.1016/j.enzmictec.2010.12.010. PMID 22112948.
- Cox, Michael; Lehninger, Albert L; Nelson, David R. (2000). Lehninger principles of biochemistry. New York: Worth Publishers. ISBN 1-57259-153-6.
- ^ Jump up to:a b Wan, P; Moat, S; Anstey, A (2011). “Pellagra: A review with emphasis on photosensitivity”. The British journal of dermatology 164 (6): 1188–200.doi:10.1111/j.1365-2133.2010.10163.x. PMID 21128910.
- Ishii, N; Nishihara, Y (1981). “Pellagra among chronic alcoholics: Clinical and pathological study of 20 necropsy cases”. Journal of neurology, neurosurgery, and psychiatry 44 (3): 209–15. doi:10.1136/jnnp.44.3.209. PMC 490893.PMID 7229643.
- Jaconello P (October 1992). “Niacin versus niacinamide”. CMAJ 147 (7): 990.PMC 1336277. PMID 1393911.
- Knip M, Douek IF, Moore WP, et al. (2000). “Safety of high-dose nicotinamide: a review”. Diabetologia 43 (11): 1337–45. doi:10.1007/s001250051536.PMID 11126400.
Food sources
“Food Data Chart – Niacin”. Retrieved 7 September 2012.
Niacin is found in variety of foods, including liver, chicken, beef, fish, cereal, peanuts and legumes, and is also synthesized from tryptophan, an essential amino acid found in most forms of protein.
Animal products:
- liver, heart and kidney (9 – 15 mg niacin per 100 grams)
- chicken, chicken breast (6.5 mg)
- beef (5 – 6 mg)
- fish: tuna, salmon, halibut (2.5 – 13 mg)
- eggs (0.1 mg)
- venison (8.43 mg)
Fruits and vegetables:
- avocados (1 mg niacin per 100 grams)
- dates (2 mg)
- tomatoes (0.7 mg)
- leaf vegetables (0.3 – 0.4 mg)
- broccoli (0.6 mg)
- carrots (0.3 – 0.6 mg)
- sweet potatoes (0.5 – 0.6 mg)
- asparagus (0.4 mg)
Seeds:
- nuts (2 mg niacin per 100 grams)
- whole grain products (4 – 29.5 mg)
- legumes (0.4 – 16 mg)
- saltbush seeds
Fungi:
- mushrooms, shiitake mushrooms (3.5 – 4 mg niacin per 100 grams)
- brewer’s yeast (36 mg)
Other:
- Monster Energy drink (40 mg per 16 ounces)
- Rockstar Energy (100% in the Super Sours flavors)
- Red Bull Energy Drink (28 mg per 12 ounces)
- Five Hour Energy drink (30 mg per 1.93 ounces)
- Ovaltine (18 mg)
- Peanut butter (15 mg)
- Tofu
- Soy sauce (0.4 mg)
- Vegemite (from spent brewer’s yeast) (110 mg niacin per 100 grams)
- Marmite (from spent brewer’s yeast) (110 mg niacin per 100 grams)












 Melting corks allow for temperature-controlled release of drugs from microscale vessels
Melting corks allow for temperature-controlled release of drugs from microscale vessels A novel nanoparticle-based drug delivery system prevents the premature release of therapeutic compounds
A novel nanoparticle-based drug delivery system prevents the premature release of therapeutic compounds