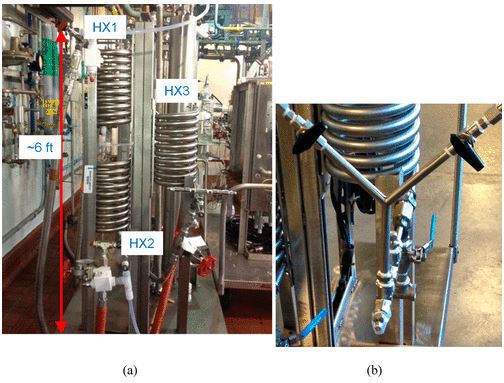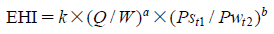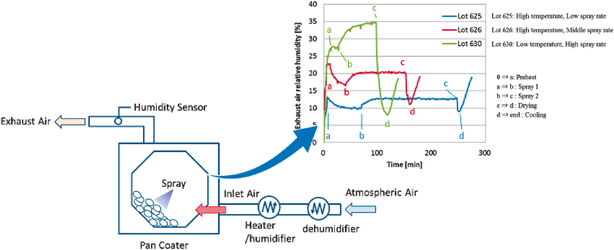
In the pharmaceutical tablet film coating process, we clarified that a difference in exhaust air relative humidity can be used to detect differences in process parameters values, the relative humidity of exhaust air was different under different atmospheric air humidity conditions even though all setting values of the manufacturing process parameters were the same, and the water content of tablets was correlated with the exhaust air relative humidity. Based on this experimental data, the exhaust air relative humidity index (EHI), which is an empirical equation that includes as functional parameters the pan coater type, heated air flow rate, spray rate of coating suspension, saturated water vapor pressure at heated air temperature, and partial water vapor pressure at atmospheric air pressure, was developed. The predictive values of exhaust relative humidity using EHI were in good correlation with the experimental data (correlation coefficient of 0.966) in all datasets. EHI was verified using the date of seven different drug products of different manufacturing scales. The EHI model will support formulation researchers by enabling them to set film coating process parameters when the batch size or pan coater type changes, and without the time and expense of further extensive testing.
EHI is defined as the following equation:
In general, pharmaceutical film coatings are applied in order to protect core tablets from light or for masking the taste of the active pharmaceutical ingredients. Therefore, the surface state of the coating layer is important to maintain the expected performance. During the coating process, however, the coating layer surface state is affected by the water content of the tablets. In a conventional approach, the water content of drug products is maintained at the validated level by monitoring the product’s temperature and/or the exhaust air temperature during the coating process. In a scale up study, the batch scale and manufacturing equipment are changed according to the progress of the process development stage. At each stage, the water content of drug products is constantly monitored and well-controlled to secure the consistency of the drug product’s quality. In this approach, numerous experiments are necessary to optimize the process parameters in each batch scale. As a result, the costs of materials, human resources, and time for development will become considerable.
A Novel Scale Up Model for Prediction of Pharmaceutical Film Coating Process Parameters
Chemical and Pharmaceutical Bulletin
Vol. 64 (2016) No. 3 p. 215-221
http://doi.org/10.1248/cpb.c15-00644
Conclusion
In this study, the relationship between film coating process parameters and EARH was clarified. In addition, it was confirmed that the EARH affected the water content of tablets. These results indicated that the water content of tablets can be regulated by controlling the EARH. From these results, we proposed the EHI for quantification of the pharmaceutical film coating process. The fitting parameters in the EHI equation were set using the experimental data of 10 drug products and 7 kinds of pan coaters. These fitting parameters of EHI were validated by evaluating the correlation coefficient determined by comparing the calculated values of EARH and the measured experimental values of EARH from various drug products, pan coater scales and coating parameters. The main advantage of the EHI method is that commercial scale coating conditions can be predicted using only one film coating experimental result from a lab-scale pan coater.
/////////pan coater, exhaust air relative humidity index (EHI), scale up, drying ability, atmospheric air, tablet water content