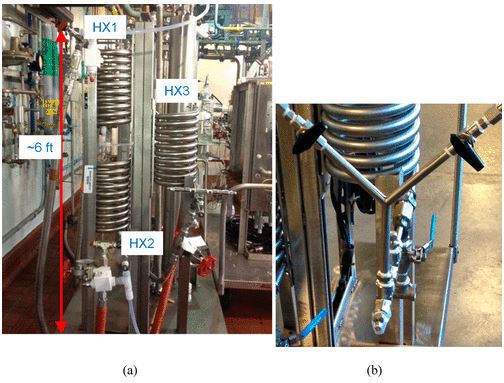
Flow reactor equipment that was used in the piloting of the aldol flow chemistry. (a) The tube-in-shell heat exchangers were used to control stream temperature upstream and downstream of the (b) Y-mixer. Valves and ports on Y-mixer enabled flushing of lines and incorporation of inline thermocouples.
Examples of continuous flow reactions in the laboratory setting are becoming commonplace in pharmaceutical drug substance research. Developing these processes for robust commercialization and identifying the scale-up parameters remains a challenge. An aldol reaction in the formation of an active pharmaceutical ingredient intermediate was developed in flow at the milliliter scale. Research focused on identifying conditions that led to robust and stable operating conditions. Desired reaction performance was achieved in various mixers across reactor scales by identifying conditions that led to similar flow regimes. Conditions from the lab were transferred to the pilot plant to successfully process ∼200 kg of the starting material.
Development and Scale-Up of a Continuous Reaction for Production of an Active Pharmaceutical Ingredient Intermediate
Conclusions
READ AT……….https://pubs.acs.org/doi/10.1021/acs.oprd.8b00192
///////////////Development, Scale-Up, Continuous Reaction, Production, Active Pharmaceutical Ingredient, Intermediate, flow chemistry, mixing sensitive reaction, scale-up
















 The presentation will load below
The presentation will load below CHEMTRIX
CHEMTRIX

