Dr. Susan Wilkinson, Deputy Editor for the European Journal of Organic Chemistry, talks to Professor Beate Koksch, Freie Universität Berlin, Germany, and Professor Peter Seeberger, Max Planck Institute of Colloids and Interfaces, Potsdam, Germany, about their article on the synthesis of fluorinated amino acids recently published in the European Journal of Organic Chemistry.
Flow Synthesis of Fluorinated α-Amino Acids
Dr. Wilkinson, European Journal of Organic Chemistry, talks to Professors Koksch and Seeberger about fluorinated amino acids
http://www.chemistryviews.org/details/ezine/7956531/Flow_Synthesis_of_Fluorinated_-Amino_Acids.html
Professor Beate Koksch, Freie Universität Berlin, Germany,
 .Prof. Dr. Beate Koksch
.Prof. Dr. Beate Koksch
Institute of Chemistry and Biochemistry – Organic Chemistry
Freie Universität Berlin
Takustr. 3
14195 Berlin
Working Group: AG Koksch
Space: 32.18
Tel .: + 49-30-838-55344, Fax -55 644
Secretariat:
Tel .: + 49-30-838-55880
(woman Skowronski, room 32.17)
Email: Beate.Koksch  fu-berlin.de
fu-berlin.de
 .
.
 |
Free University of Berlin |
Nominated by
- German Research Foundation (DFG)
- AcademiaNet member since 13.03.2015
Employed by
- Freie Universität Berlin
Academic Discipline/Fields
- Natural sciences/ Engineering/ Agricultural sciences
Field
Chemistry
Area of specialisation
Organic and Natural Product Chemistry
Research interests
- folding mechanisms occuring in neurodegenerative diseases
- developing new multivalent scaffolds
- investigating the impact of fluorine on amino acids, peptides and proteins
Distinctions and Awards
- Georg Thieme publisher’s award, 2002Lessing medal in gold, 1986
………………………………………………..
Professor Peter Seeberger, Max Planck Institute of Colloids and Interfaces, Potsdam, Germany

Since 2011, Professor Peter H. Seeberger, Max Planck Institute of Colloids and Interfaces in Potsdam, Germany, is Editor-in-Chief of the Beilstein Journal of Organic Chemistry.
Editor-in-Chief of the Beilstein Journal of Organic Chemistry is Professor Peter H. Seeberger, Max Planck Institute of Colloids and Interfaces in Potsdam, Germany, who is supported by a distinguished board of associate editors, each of whom is responsible for a particular subject area within the journal’s scope. Over 40 scientists from all over the world, including several Nobel Prize laureates, support the Beilstein Journal of Organic Chemistry as Advisory Board members.
Prof. Dr. Peter H. Seeberger
German researchers develop cheap and high-yield process to manufacture anti-malaria drug
Researchers at the Max Planck Institute of Colloids and Interfaces in Potsdam and the Freie Universität Berlin have developed a very simple process for the synthesis of artemisinin – the best anti-malaria drug – more economically and in sufficient volumes for all patients. This means that it will be possible to provide medication for the 225 million malaria patients in developing countries at an affordable price.
 An Anopheles female mosquito that transmits malaria(© picture alliance/dpa Fotografia)Over one million people die of malaria each year because they do not have access to effective drugs.Millions, especially in the developing world, cannot afford the combination drug preparation, which consists mainly of artemisinin.
An Anopheles female mosquito that transmits malaria(© picture alliance/dpa Fotografia)Over one million people die of malaria each year because they do not have access to effective drugs.Millions, especially in the developing world, cannot afford the combination drug preparation, which consists mainly of artemisinin.
Moreover, the price for the medication varies, as this substance is isolated from sweet wormwood (Artemisia annua) which grows mainly in China and Vietnam, and varies seasonally in its availability.
Pharmaceutical companies could only obtain the drug from plants up to now. The chemists use a waste product from current artemisinin production as their starting substance. This substance can also be produced biotechnologically in yeast, which the scientists convert into the active ingredient using a simple yet very ingenious method.
This may be about to change. Peter H. Seeberger, Director at the Max Planck Institute of Colloids and Interfaces in Potsdam and Professor of Chemistry at the Freie Universität Berlin and his colleague François Lévesque have discovered a very simple way of synthesising the artemisinin molecule, which is known as an anti-malaria drug from traditional Chinese medicine and has an extremely complex chemical structure. “The production of the drug is therefore no longer dependent on obtaining the active ingredient from plants,” says Peter Seeberger.
Synthesis from a by-product of artemisinin production
As a starting point, the chemists use artemisinic acid – a substance produced as a hitherto unused by-product from the isolation of artemisinin from sweet wormwood, which is produced in volumes ten times greater than the active ingredient itself. Moreover, artemisinic acid can easily be produced in genetically modified yeast as it has a much simpler structure. “We convert the artemisinic acid into artemisinin in a single step,” says Peter Seeberger. “And we have developed a simple apparatus for this process, which enables the production of large volumes of the substance under very controlled conditions.”
The effect of the molecule, which not only targets malaria but possibly also other infections and even breast cancer, is due to, among other things, a very reactive chemical group formed by two neighbouring oxygen atoms – which chemists refer to as an endoperoxide. Peter Seeberger and François Lévesque use photochemistry to incorporate this structural element into the artemisinic acid. Ultraviolet light converts oxygen into a form that can react with molecules to form peroxides.
800 photoreactors should suffice to cover the global requirement for artemisinin
 Dr. Peter H. Seeberger, Director at the Max Planck Institute of Colloids and Interfaces in Potsdam and Professor of Chemistry at the Freie Universität Berlin(© dpa)“Photochemistry is a simple and cost-effective method. However, the pharmaceutical industry has not used it to date because it was so difficult to control and implement on a large scale,” explains Peter Seeberger.
Dr. Peter H. Seeberger, Director at the Max Planck Institute of Colloids and Interfaces in Potsdam and Professor of Chemistry at the Freie Universität Berlin(© dpa)“Photochemistry is a simple and cost-effective method. However, the pharmaceutical industry has not used it to date because it was so difficult to control and implement on a large scale,” explains Peter Seeberger.
“The fact that we do not carry out the synthesis as a one-pot reaction in a single vessel, but in a continuous-flow reactor enables us to define the reaction conditions down to the last detail,” explains Peter Seeberger.
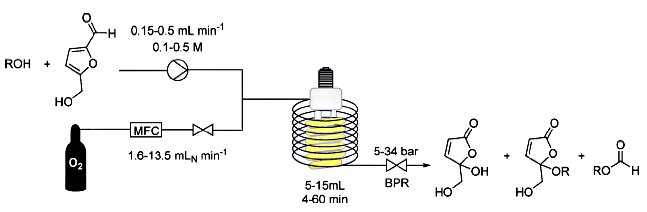
After just four and a half minutes a solution flows out of the tube, in which 40 percent of the artemisinic acid has become artemisinin. “We assume that 800 of our simple photoreactors would suffice to cover the global requirement for artemisinin,” says Peter Seeberger. And it could all happen very quickly. Peter Seeberger estimates that the innovative synthesis process could be ready for technical use in a matter of six months. This would alleviate the global shortage of artemisinin and exert considerable downward pressure on the price of the associated drugs…….see http://www.india.diplo.de/Vertretung/indien/en/__pr/Edu__Science__News/Malaria__drug.html
Max Planck Institute for Colloids and Interfaces
Peter Seeberger
Department of Biomolecular Systems
Max Plank Institute for Colloids and Interfaces
(Potsdam, Germany)
peter.seeberger@mpikg.mpg.de
http://www.peter-seeberger.de/
The core interests our research program currently address the following areas:
Automated oligosaccharide synthesis
- Rapid access to monosaccharide by de-novo synthesis
- New protecting groups
- New Glycosylating Agents
- New linkers for solid phase carbohydrate synthesis
- Assembly of complex structures (in particular N-Glycans, O-Glycans)
- Optimization of steps followingthe assembly, like deprotection, modification and conjugation
Total Synthesis of Biologically Important Oligosaccharides
- Tumor-associated antigens
- HIV-related oligosaccharides
- Bacterial cell-surface antigens
- N-linked glycoproteins
Chemical Synthesis and Biochemistry of Proteoglycans
- Modular synthesis of heparin/heparan sulfates
- Creation of heparin microarray
- Optimization of the building blocks synthesis
- Study of the SAR (structure-activity relationship) and the interactions between Proteoglycans and proteins
- Automated synthesis of heparin fragments
Total Synthesis and Biological Activity of Glycosylphosphatidylinositols (GPIs)
- Total syntheses of GPIs
- Development of a synthetic GPI malarial vaccine
- Elucidation of the biosynthesis of GPI
- Immunological response to synthetic GPIs
Development of Cabohydrate-based Vaccines
- A fully synthetic malaria vaccine
- Leishmania vaccine
- Synthetic HIV vaccine
- Synthetic TB vaccine
Microreactors for Organic Synthesis
- (Automated) Synthesis in continuous flow Microreactors
- Photochemistry in Microflow reactors
- Catalysis in Microreactors
Carbohydrate Microarrays
De novo synthesis
Nanoparticules and Colloidal Polymers
- Quantum dots
- Supramolecular dendrimers
- Emulsion polymerization of nanoparticules


 http://www.theguardian.com/technology/2012/feb/05/malaria-drug-synthesis-peter-seeberger
http://www.theguardian.com/technology/2012/feb/05/malaria-drug-synthesis-peter-seeberger
 .
.
take a tour
Potsdam, Germany
-
Potsdam – Wikipedia, the free encyclopedia
en.wikipedia.org/wiki/PotsdamPotsdam (German pronunciation: [ˈpɔtsdam] ( listen)), is the capital city of the German federal state of Brandenburg. It directly borders the German capital Berlin …










//////////////


















 Charlotte
Charlotte 










 Floris P.J.T. Rutjes
Floris P.J.T. Rutjes