Palladium-Catalyzed Fluoride-Free Cross-Coupling of Intramolecularly ActivatedAlkenylsilanes and Alkenylgermanes: Synthesis of Tamoxifen as a Synthetic Application (pages 642–650)Kenji Matsumoto and Mitsuru ShindoArticle first published online: 23 FEB 2012 | DOI: 10.1002/adsc.201100627

http://pubs.rsc.org/en/content/articlelanding/2011/cs/c0cs00129e#!divAbstract

EP 0883587 A1 WO1997026234A1)
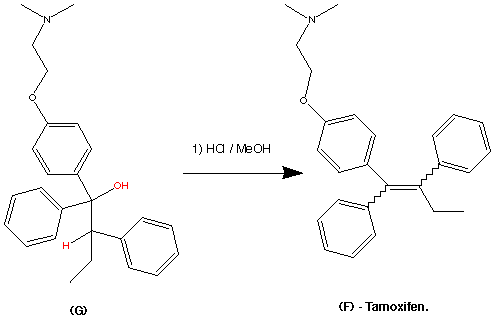
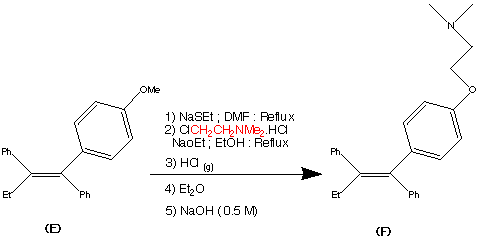
Preparation of Z isomer of Tamoxifen
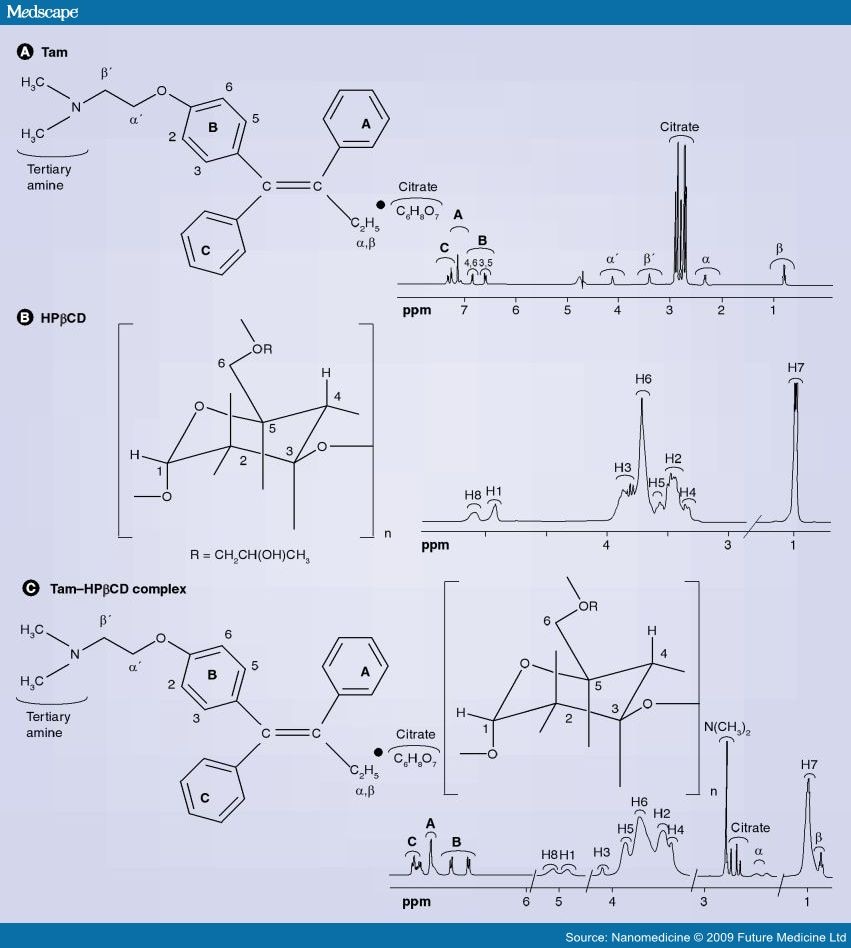
A solution of bromobenzene (3.92g, 25mmol) in ether (5ml) containing a crystal of iodine was added dropwise to a suspension of magnesium turnings (0.63g, 26mmol) in ether (5ml) at reflux, under nitrogen. After the addition was complete, the reaction mixture was cooled to room temperature and a solution of l- [ 4- ( 2- chloroethoxy)phenyl]-2-phenyl-l-butanone (3.75g, 12.4mmol) in ether (15ml) was added over 1 hour. The resulting mixture was refluxed for 16 hours, then poured into dilute hydrochloric acid (50ml) and extracted with ether (3x40ml) . The combined ether layers were concentrated, the residual oil was dissolved in ethanol (10ml) and refluxed with concentrated hydrochloric acid (5ml) for 4 hours. The organic phase was separated, dried (Na2S04) and evaporated to dryness to give a yellow oil. Η NMR (see Figures 1 to 4 and discussion below) showed this to be a 2:1 mixture of the Z and E isomers. The oil was then dissolved in warm methanol (about 40°C) and allowed to cool to room temperature. The colourless crystals formed proved to be pure Z isomer of 2-chloroethoxy tamoxifen (4.12g, 11.4mmol, 92% yield) . M.p. 107-109°C, m/z 362/364 (chlorine atom present), <SH 0.92 (3H, t, J = 7.33 Hz, CH3) , 2.46 (2H, q, J = 7.33 Hz, CH2CH3) , 3.72 (2H, t, J = 5.86 Hz, 0CH2CH2C1) , 4.09 (2H, t, J = 5.86 Hz, 0CH2CH2C1) , 6.55 (2H, d, J = 8.79 Hz, aromatic protons ortho to 0CH2CH2C1) , 6.79 (2H, d, J = 8.79 Hz, aromatic protons meta to 0CH2CH2C1) , 7.10-7.38 (10H, m, the two remaining C6H5 ,s) (see Figure 5) . The 2-chloroethoxy tamoxifen was reacted with dimethylamine in ethanol, under reflux, to produce the desired Z isomer of tamoxifen.
Analysis of Η NMR data
Figures 1 to 4 represent a mixture of the E- and Z- forms of compound XI described above.
The expansion of the region ό* 0.80 to 1.05 shows two overlapping triplets corresponding to the CH3 groups in the
Z- and E- derivatives respectively. The critical point is the ratio of the heights of the peaks at 0.92 (for the Z) and 0.94 (for the E) , which is approximately 2:1. The expansion of the 4.00 to 4.35 region reveals similar information where ratios are 10:6.4 and 5.56:3.43.
Similarly expansion of the region 3.6 to 3.9 shows the ratio to be 2.46:1. All of these measurements suggest an approximate 2:1 ratio.
Referring to Figure 5, this shows almost pure Z- isomer. It should be noted that there is 660 mg of this from an original mixture of a 2:1 ratio mixture of 780 mg which would contain only 520 mg of the Z-isomer.

Z isomer of tamoxifen and 4-hydroxytamoxi en include stereoselective syntheses (involving expensive catalysts) as described in J. Chem. Soc, Perkin Trans I 1987, 1101 and J. Org. Chem. 1990, 55, 6184 or chromatographic separation of an E/Z mixture of isomers as described in J. Chem. Res., 1985 (S) 116, (M) 1342, 1986 (S) 58, (M) 771.
(Z)-tamoxifen (1) as a white solid, mp: 95.8-96.3 ºC. 1H-NMR (500 MHz, CDCl3) d 0.92 (3H, t, J 7.3 Hz), 2.29 (6H, s), 2.45 (2H, q, J 7.3 Hz), 2.65 (2H, t, J 5.8 Hz), 3.93 (2H, t, J 5.8 Hz), 6.68 (2H, d, J 9.5 Hz), 6.78 (2H, d, J 9.5 Hz), 7.08-7.28 (10H, m).13C-NMR (125 MHz, CDCl3) d 13.6 (CH3), 29.0 (CH2), 45.8 (CH3), 58.2 (CH2), 65.5 (CH2), 113.4 (C), 126.0 (C), 126.5 (CH), 127.8 (CH), 128.1 (C), 129.7 (C), 131.8 (CH), 135.6 (CH), 138.2 (CH), 141.3 (CH), 142.4 (CH), 143.8 (C), 156.7 (C). IR (KBr film) nmax/cm-1: 3055, 2979, 2925, 2813, 2769, 1606, 1509, 1240, 1035, 707. GC–MS (EI) m/z 371(5%), 58(100%).
(Z)-tamoxifen (1) and (E)-tamoxifen (2) in 52% yield. 1H-NMR (300 MHz, CDCl3) d 0.91 (Z isomer. 3H, t, J 7.3 Hz), 0.94 (E isomer. 3H, t, J 7.3 Hz), 2.28 (Z isomer. 6H, s), 2.34 (E isomer. 6H, s), 2.42-2.52 (Z and Eisomers. 4H, m), 2.63 (Z isomer. 2H, t, J 5.9 Hz), 2.74 (E isomer. 2H, t, J 5.9 Hz), 3.94 (Z isomer. 2H, t, J 5.9 Hz), 4.07 (E isomer. 2H, t, J 5.9 Hz), 6.68 (Z isomer. 2H, d, J 9.7 Hz), 6.76 (E isomer. 2H, d, J 9.3 Hz), 6.86-7.36 (Z and E isomers. 10H, m). IR (KBr film) nmax/cm-1: 3081, 3056, 2974, 2826, 2770, 1611, 1509, 1238, 1044. GC–MS (EI) m/z: Z isomer, 371(4%), 72 (24%), 58(100%); E isomer, 371(3%), 72 (24%), 58(100%). (the diastereoisomeric ratio was determined by capillary GC analysis and the configuration of the major diastereoisomer established by comparison of the NMR data of the synthetic mixture with an authentic sample of (Z)-tamoxifen (1).
nmr
ir
FTIR
shows the typical spectra’s of pure tamoxifen citrate, PCL, a physical mixture of tamoxifen citrate and PCL and drug-loaded implants. The spectrum of tamoxifen citrate shows characteristic absorption bands at 3027 cm−1 (=C-H stretching), 1507 and 1477 (C=C ring stretching) and 3180 cm -1 (-NH2). PCL displays a characteristic absorption band at strong bands such as the carbonyl stretching mode around 1727 cm−1 (C=O), asymmetric stretching 2949 cm−1 (CH 2 ) symmetric stretching 2865 cm−1 (CH 2 ). No changes in the spectrum of the physical mixture and drug-loaded microspheres were evident by FTIR spectroscopy. The strong bands such as the carbonyl peak were clear at all points.
 |
Figure 2: Transmission FTIR spectra of (a) tamoxifen-loaded implant, (b) physical mixture of drug+PCL, (c) pure PCL, (d) pure tamoxifen citrate |
enlarged view

FTIR spectra of A) tamoxifen citrate; B) PLGA; C) mixture of drug and excipients; D) freshly prepared nanoparticles in the formulation (BS-3HS).
Mentions: The pure drug tamoxifen citrate, PLGA-85:15, PVA, a mixture of PLGA and PVA, and a mixture of tamoxifen citrate, PLGA, and PVA; and a freshly prepared formulation were mixed separately with IR grade KBr in the ratio of 1:100 and corresponding pellets were prepared by applying 5.5 metric ton pressure with a hydraulic press. The pellets were scanned in an inert atmosphere over a wave number range of 4000–400 cm−1 in Magna IR 750 series II, FTIR instrument (Nicolet, Madison, WI, USA).
dsc

DSC thermograms of pure tamoxifen (a), pure PCL (b), physical mixture of drug+PCL (c) and (d) drug-loaded implant. The experiment was carried with crimped aluminum pans and a heating rate of 10ºC/min
xrd

X-ray diffraction studies of pure drug (a), pure PCL (b), physical mixture of drug+PCL (c) and (d) drug-loaded implant
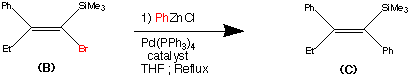
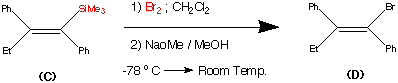
synthesis
J.Chem. Research,1985(S) 116, (M) 1342 and 1986 (S) 58, (M) 0771.


































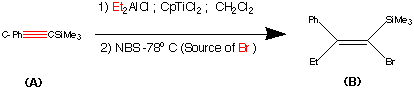























 Copper-based radiopharmaceuticals for diagnostic imaging can target the amyloid-β plaques implicated in Alzheimer’s disease
Copper-based radiopharmaceuticals for diagnostic imaging can target the amyloid-β plaques implicated in Alzheimer’s disease
