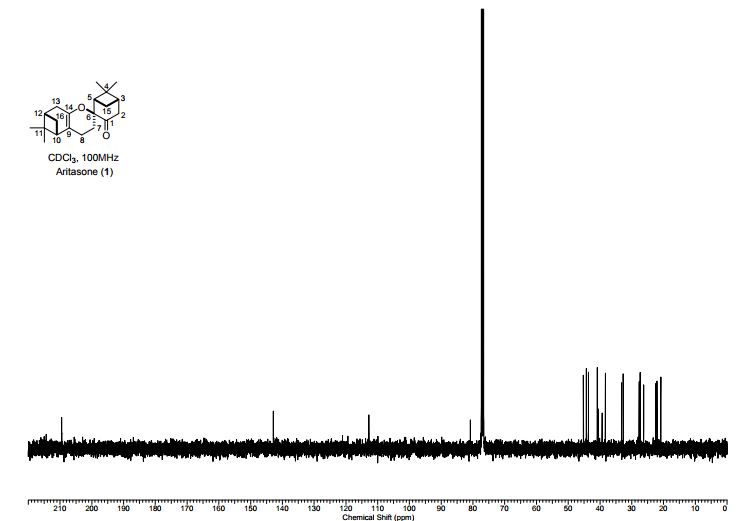
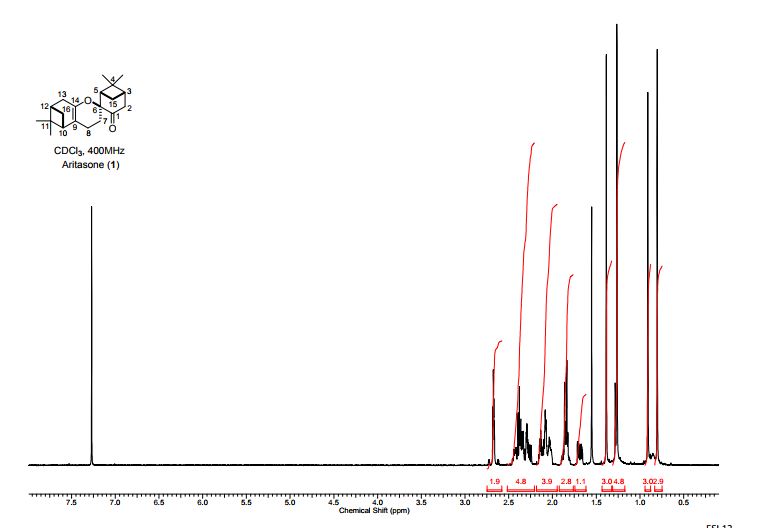
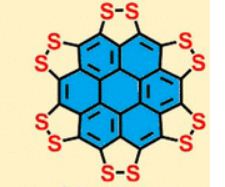
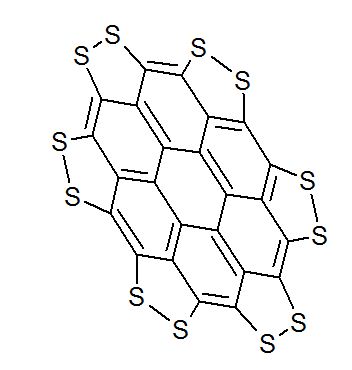
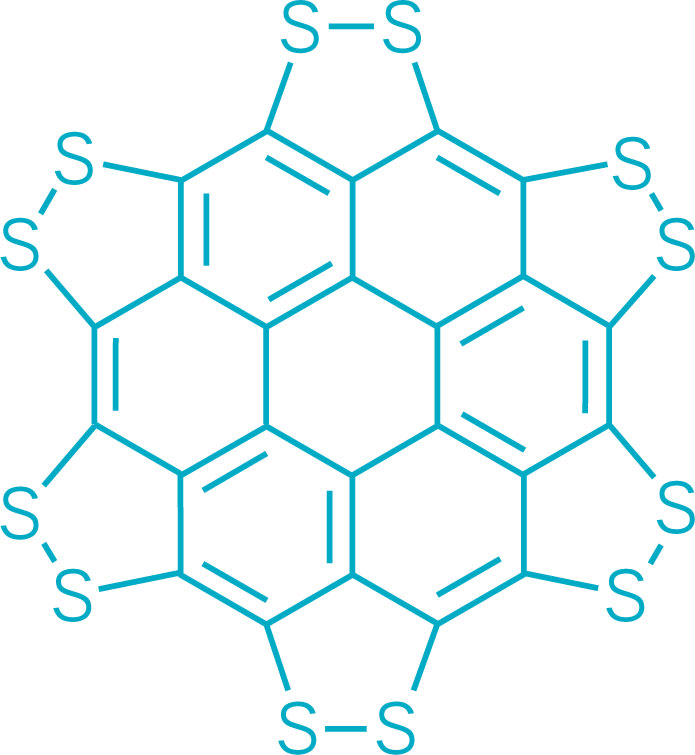
A persulfurated coronene, a molecule dubbed a “sulflower” for its resemblance to a sunflower, bloomed this year. It’s the first fully sulfur-substituted polycyclic aromatic hydrocarbon and only the second member of a new class of circular heterocyclic carbon sulfide compounds, after the synthesis of octathio[8]circulene a decade ago.
Chemists hope to create other class members, including the simplest one, persulfurated benzene, for use in battery cathodes and other electronic materials.
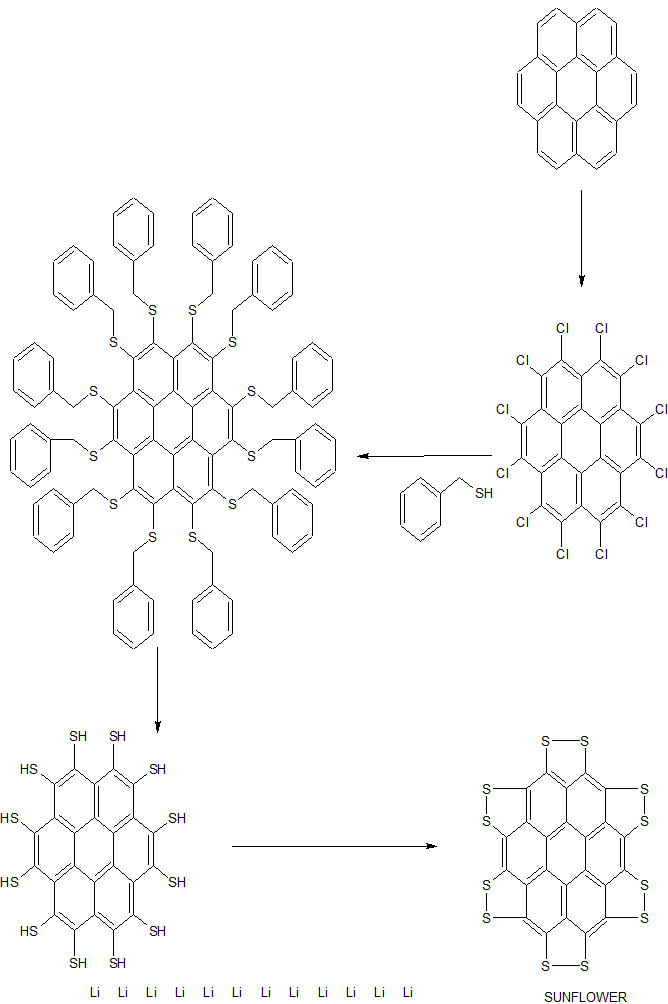
A team led by Xinliang Feng of Dresden University of Technology and Klaus Müllen of the Max Planck Institute for Polymer Research created the sulflower (J. Am. Chem. Soc. 2017, DOI: 10.1021/jacs.6b12630).
http://pubs.acs.org/doi/abs/10.1021/jacs.6b12630
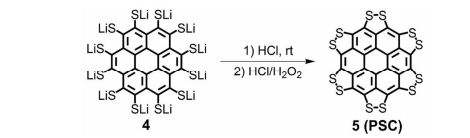
Synthesis of persulfuratedcoronene (5, PSC)
5 (82 mg) as dark red solid in 61% yield. HR-MS (HR-MALDI-TOF) m/z: Calcd. for C24S12: 671.6629; Found 671.6648 [M]+; Elem. Anal. calcd. for C24S12: C, 42.83; S, 57.17. Found: C, 42.87; S, 57.13.
Persulfurated Coronene: A New Generation of “Sulflower”

We report the first synthesis of a persulfurated polycyclic aromatic hydrocarbon (PAH) as a next-generation “sulflower.” In this novel PAH, disulfide units establish an all-sulfur periphery around a coronene core. The structure, electronic properties, and redox behavior were investigated by microscopic, spectroscopic and electrochemical methods and supported by density functional theory. The sulfur-rich character of persulfurated coronene renders it a promising cathode material for lithium–sulfur batteries, displaying a high capacity of 520 mAh g–1 after 120 cycles at 0.6 C with a high-capacity retention of 90%
Renhao Dong

Research Group Leader
Renhao received his PhD in Physical Chemistry from Shandong University in 2013. Since 01/2017, he is a research group leader at the Chair for Molecular Functional Materials in TUD. His current research interest focuses on synthesis of organic 2D crystals (2D polymers/COFs/MOFs) and their applications in electronics and energy technology.
Contact
Phone: +49 – 351 / 463-40401 or -34932
Email: renhao.dong@tu-dresden.de

Prof. Xinliang Feng
Work Biography:
This is a professorship in the context of the cluster of excellence cfaed.
Xinliang Feng received his Bachelor’s degree in analytic chemistry in 2001 and Master’s degree in organic chemistry in 2004. Then he joined Prof. Klaus Müllen’s group at the Max Planck Institute for Polymer Research for PhD thesis, where he obtained his PhD degree in April 2008. In December 2007 he was appointed as a group leader at the Max-Planck Institute for Polymer Research and in 2012 he became a distinguished group leader at the Max-Planck Institute for Polymer Research.
His current scientific interests include graphene, two-dimensional nanomaterials, organic conjugated materials, and carbon-rich molecules and materials for electronic and energy-related applications. He has published more than 370 research articles which have attracted more than 25000 citations with H-index of 75.
He has been awarded several prestigious prizes such as IUPAC Prize for Young Chemists (2009), Finalist of 3rd European Young Chemist Award, European Research Council (ERC) Starting Grant Award (2012), Journal of Materials Chemistry Lectureship Award (2013), ChemComm Emerging Investigator Lectureship (2014), Highly Cited Researcher (Thomson Reuters, 2014, 2015 and 2016), Fellow of the Royal Society of Chemistry (FRSC, 2014). He is an Advisory Board Member for Advanced Materials, Journal of Materials Chemistry A, ChemNanoMat, Energy Storage Materials, Small Methods and Chemistry -An Asian Journal. He is also one of the Deputy Leaders for European communitys pilot project Graphene Flagship, Head of ESF Young Research Group “Graphene Center Dresden”, and Working Package Leader of WP Functional Foams & Coatings of GRAPHENE FLAGSHIP.
Academic Employment
- 12/2007-12/2012: Group Leader, Max Planck Institute for Polymer Research in Mainz, Germany
- 06/2010: Director of the Institute of Advanced Organic Materials, Shanghai Jiao Tong University
- 03/2011: Distinguished Adjunct Professorship in Shanghai Jiao Tong University, Chin
- 12/2012-07/2014: Distinguished Group Leader, Max Planck Institute for Polymer Research in Mainz, Germany
- 08/2014: W3 Chair Professor, Technische Universität Dresden, Germany
Honors and Duties
- Marie Currie Fellowship (2005-2006)
- Chinese Government Award for Outstanding Self-financed Students (2008)
- IUPAC Prize for Young Chemists (2009)
- Finalist of 3rd European Young Chemist Award (2010)
- ISE (International Society of Electrochemistry) Young Investigator Award (2011)
- Adjunct Professorship, China University of Geosciences (Wuhan) (2011)
- Deputy Leader of one of the ten European representatives of the European community’s pilot project GRAPHENE FLAGSHIP (2012)
- EU FET Young Explorer (2012)
- ERC Starting Grant Award (2012)
- Advisory Board Member for Advanced Materials (2013)
- Journal of Materials Chemistry Lectureship Award (2013)
- Advisory Board Member for Journal of Materials Chemistry A (2014)
- Editorial Board Member of Chemistry – An Asian Journal (2014)
- ChemComm Emerging Investigator Lectureship (2014)
- Highly Cited Researcher (Thomson Reuters, 2014)
- Fellow of the Royal Society of Chemistry (2014)
- Highly Cited Researcher (Chemistry and Materials Science) (2015)
- International Advisory Board of Energy Storage Materials (2015)
- International Advisory Board of ChemNanoMat (2015)
- Highly Cited Researcher (Chemistry and Materials Science, Thomson Reuters) (2016)
- Head of ESF Young Research Group “Graphene Center Dresden” (2016)
- Working Package Leader of WP Functional Foams & Coatings of GRAPHENE FLAGSHIP (2016)
- International Advisory Board of Small Methods (2016)
- Path Leader of 2.5D path within the cluster of excellence CFAED (2016)
- ERC Proof-of-Concept Project Award (2017)
- Small Young Innovator Award (2017)
- Hamburg Science Award (2017)
Referee for:
Nature, Science, Nature Materials, Nature Nanotechnology, Nature Chemistry, Journal of the American Chemical Society, Angewandte Chemie International Edition, Nano Letters, Advanced Materials, Chemical Society Reviews, ACS Nano, Small, Chemical Communications, Chemistry of Materials, Organic Letters, Journal of the Organic Chemistry, Chemistry – A European Journal, ChemSusChem, ChemPhysChem, Macromolecular Rapid Communications, Journal of Material Chemistry, New Journal of Chemistry, Chemistry – An Asian Journal, ACS Applied Materials & Interfaces, Energy & Environmental Science, Organic Electronics and so on
Referee for research grants in NSF, US Department of Energy, ESF, ISF and Fondazione Cariparo and Fondazione CariModena.
Publications
Click to open publications list
Contact (Secretariat)
Phone: +49 351 / 463-43251
Fax: +49 351 / 463-43268
Email: sabine.strecker@tu-dresden.de
Max-Planck-Institute for Polymer Research, Mainz, 55128, Germany

Research into energy technologies and electronic devices is strongly governed by the available materials. We introduce a synthetic route to graphenes which is based upon the cyclodehydrogenation (“graphitization”) of well-defined dendritic (3D) polyphenylene precursors. This approach is superior to physical methods of graphene formation such as chemical vapour deposition or exfoliation in terms of its (i) size and shape control, (ii) structural perfection, and (iii) processability (solution, melt, and even gas phase). The most convincing case is the synthesis of graphene nanoribbons under surface immobilization and in-situ control by scanning tunnelling microscopy.
Columnar superstructures assembled from these nanographene discs serve as charge transport channels in electronic devices. Field-effect transistors (FETs), solar cells, and sensors are described as examples.
Upon pyrolysis in confining geometries or “carbomesophases”, the above carbon-rich 2D- and 3D- macromolecules transform into unprecedented carbon materials and their carbon-metal nanocomposites. Exciting applications are shown for energy technologies such as battery cells and fuel cells. In the latter case, nitrogen-containing graphenes serve as catalysts for oxygen reduction whose efficiency is superior to that of platinum.
Müllen, K., Rabe, J.R., Acc. Chem. Res. 2008, 41, (4), 511-520;
Wang, X., Zhi, L., Müllen, K. Nano. Lett. 2008, 8, 323-327;
Feng, X.; Chandrasekhar, N.; Su, H. B.; Müllen, K., Nano. Lett. 2008, 8, 4259.;
Pang, S.; Tsao, H. N.; Feng, X.; Müllen, K., Adv. Mater. 2009, 31, 3488;
Feng, X., Marcon, V., Pisula, W., Hansen, M.R., Kirkpatrick, I., Müllen, K., Nature Mater. 2009, 8, 421;
Cai, J., Ruffieux, P., Jaafar, R., Bieri, M., Braun, T., Blankenburg, S., Muoth, M., Seitsonen, A. P., Saleh, M., Feng, X., Müllen, K., Fasel, R., Nature 2010, 466, 470-473;
Yang, S., Feng, X., Zhi, L., Cao, Q., Maier, J., Müllen, K., Adv. Mater. 2010, 22, 838; Liu, R., Wu, D., Feng, X., Müllen, K., Angew. Chem. Int. Ed. 2010, 49, 2565;
Käfer, D., Bashir, A., Dou, X., Witte, G., Müllen, K., Wöll, C., Adv. Mater. 2010, 22, 384;
Diez-Perez, I., Li, Z., Hihath, J., Li, J., Zhang, C., X., Zang, L., Dai, Y., Heng, X., Müllen, K., Tao, N. J. Nature Commun. 2010, DOI: 10.1038.
Prof. Dr. Klaus Müllen
joined the Max-Planck-Society in 1989 as one of the directors of the Max-Planck Institute for Polymer Research. He obtained a Diplom-Chemiker degree at the University of Cologne in 1969 after work with Professor E. Vogel. His Ph.D. degree was granted by the University of Basel, Switzerland, in 1972 where he undertook research with Professor F. Gerson on twisted pi-systems and EPR spectroscopic properties of the corresponding radical anions. In 1972 he joined the group of Professor J.F.M. Oth at the Swiss Federal Institute of Technology in Zürich where he worked in the field of dynamic NMR spectroscopy and electrochemistry. He received his habilitation from the ETH Zürich in 1977 and was appointed Privatdozent. In 1979 he became a Professor in the Department of Organic Chemistry, University of Cologne, and accepted an offer of a chair in Organic Chemistry at the University of Mainz in 1983. He received a call to the University of Göttingen in 1988.
////////////////////
S1Sc6c8c1c9SSc%10c2SSc%13c2c%11c4c3c%13SSc3c%12SSc7c%12c4c(c5c7SSc56)c8c%11c9%10
http://pubs.acs.org/doi/abs/10.1021/jacs.6b12630






































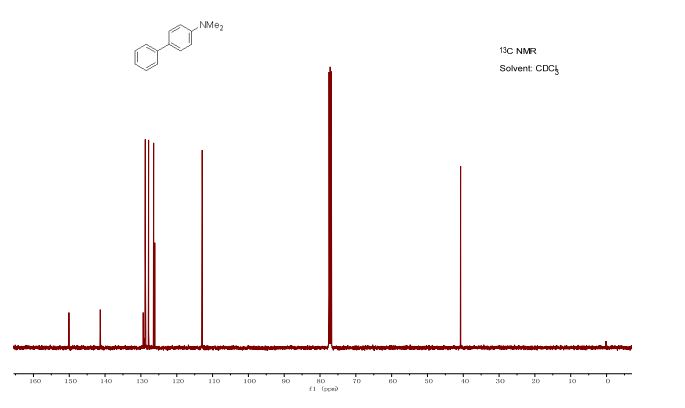

 学 系
学 系
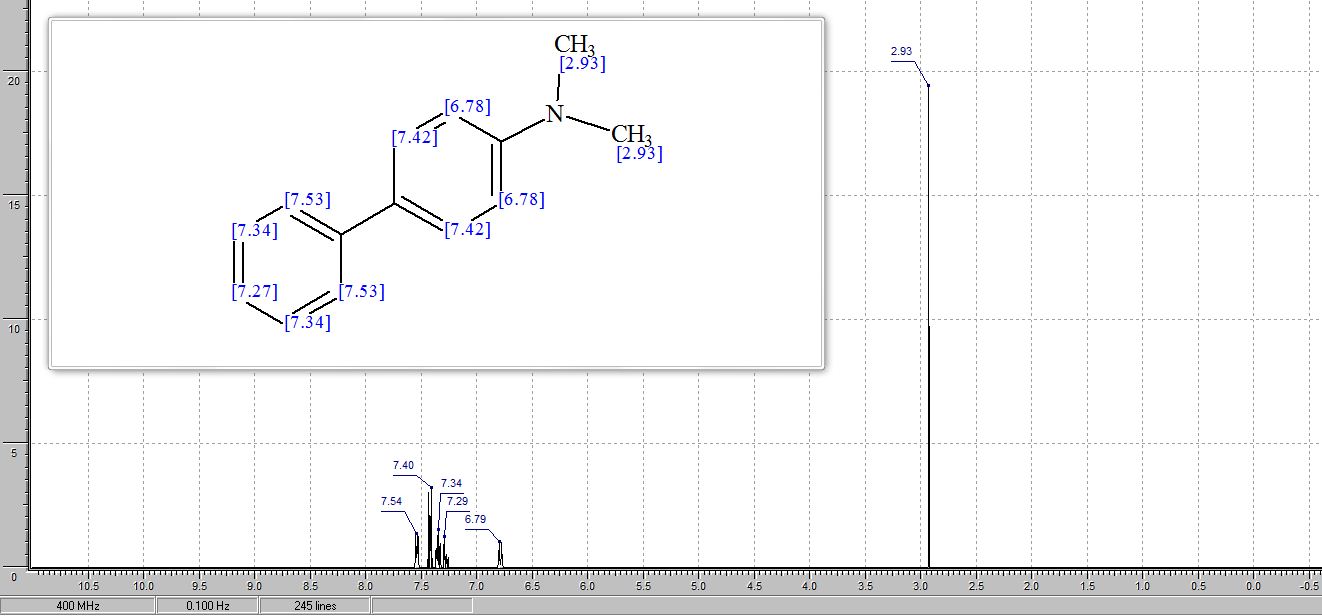
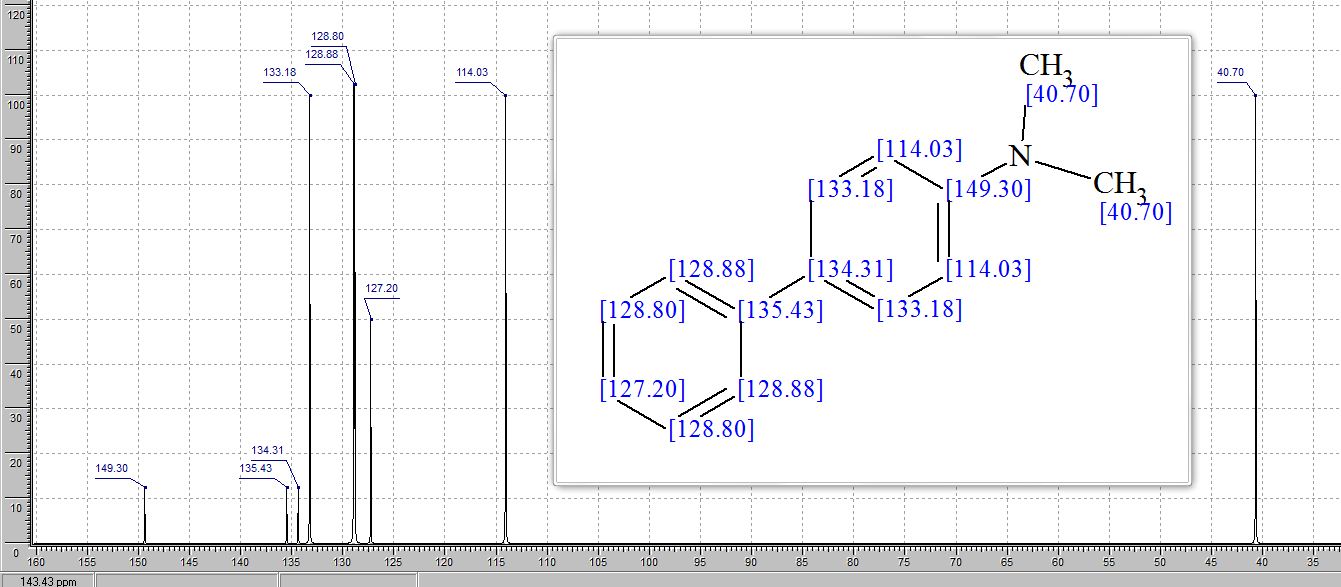

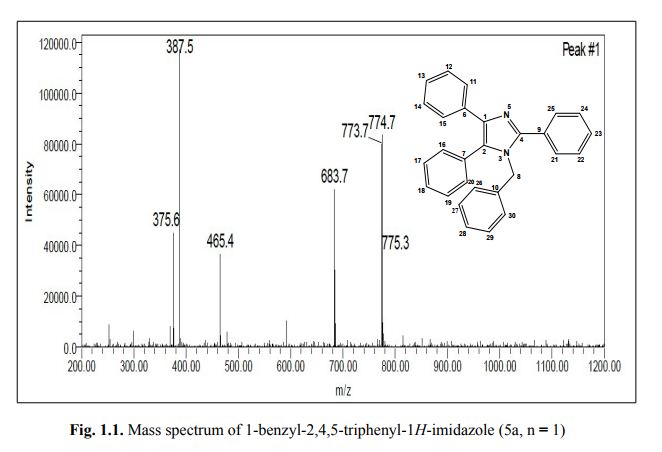
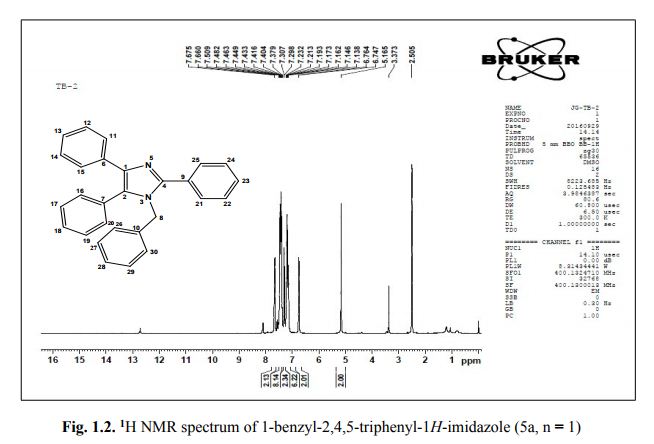
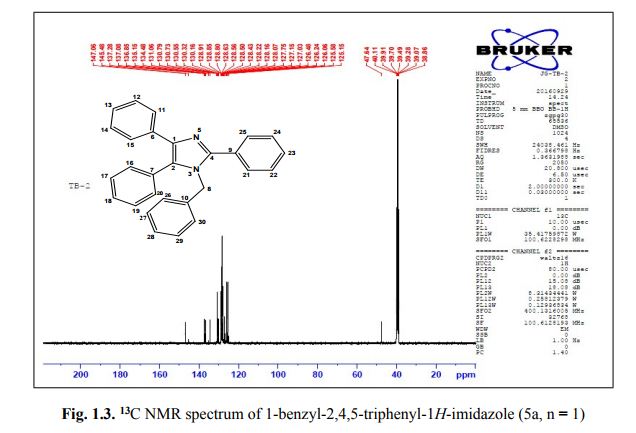


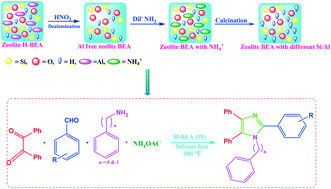
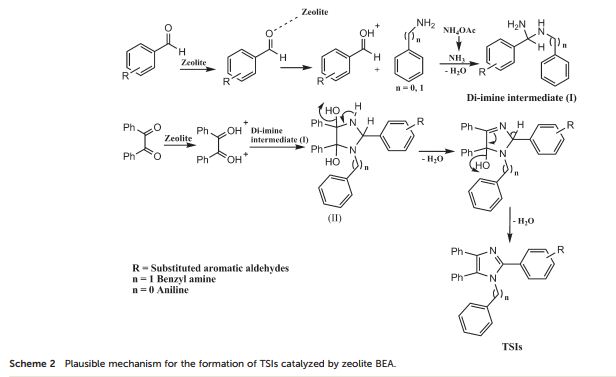
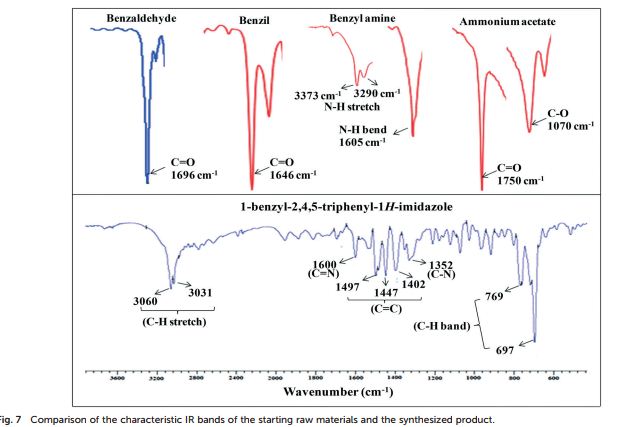







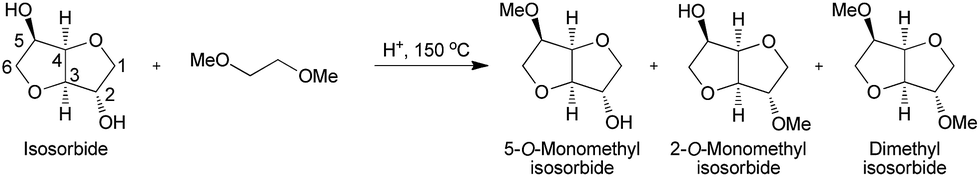
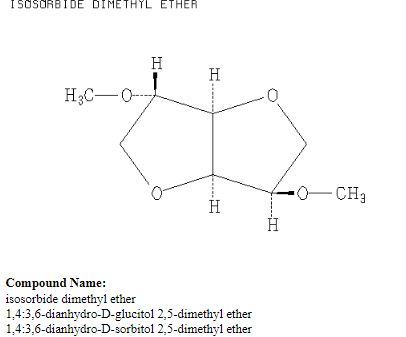
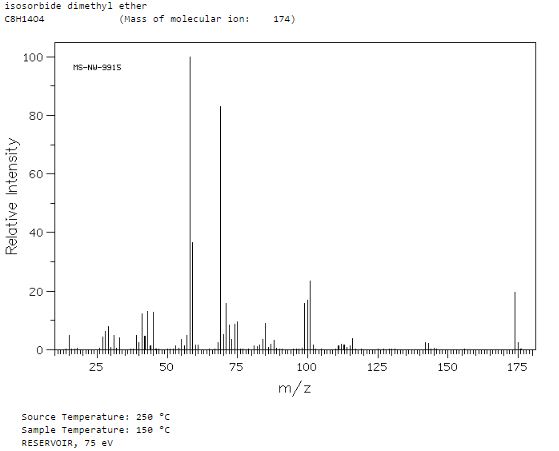
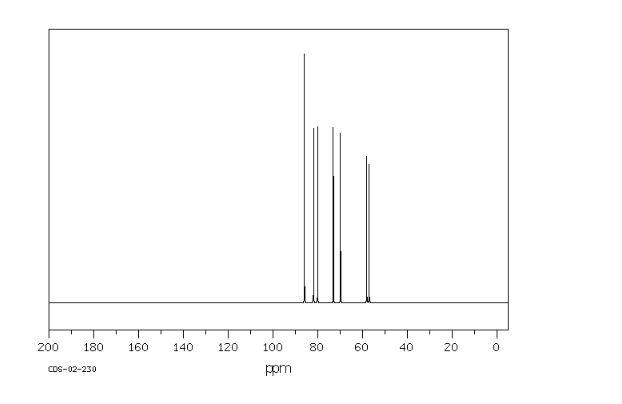
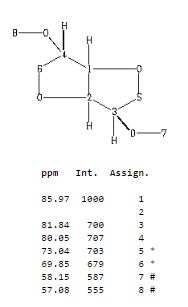
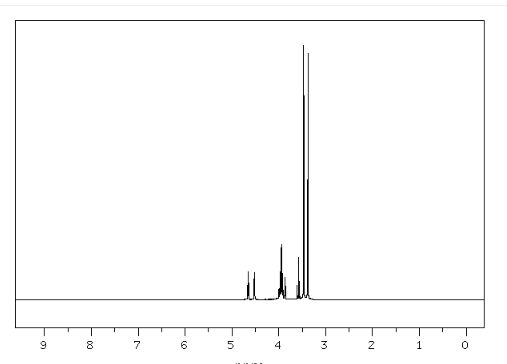
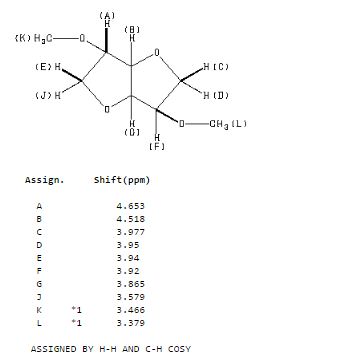
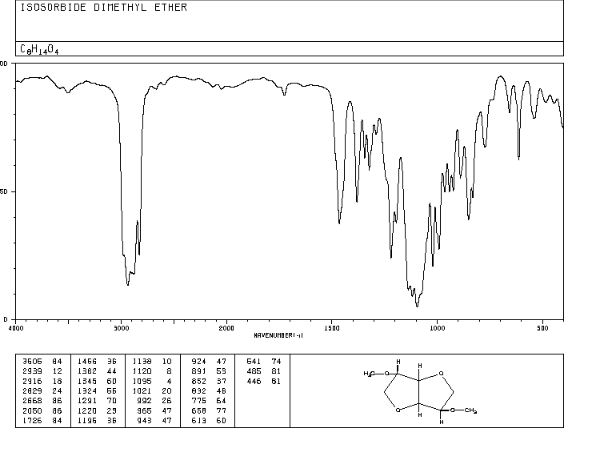
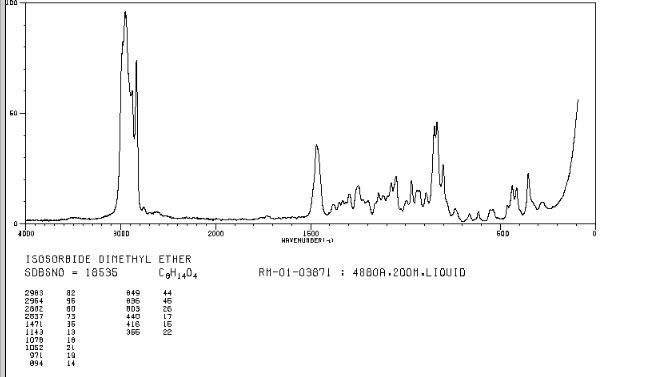
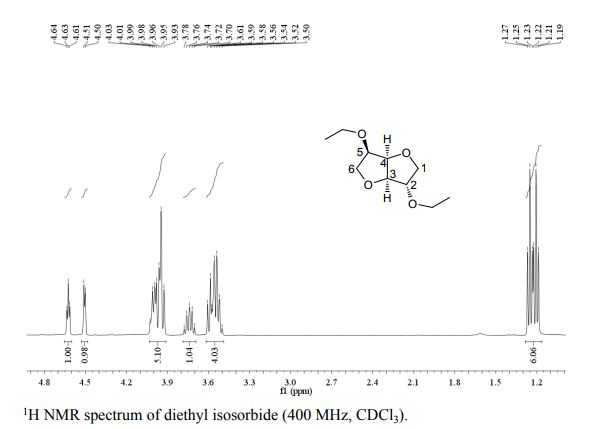
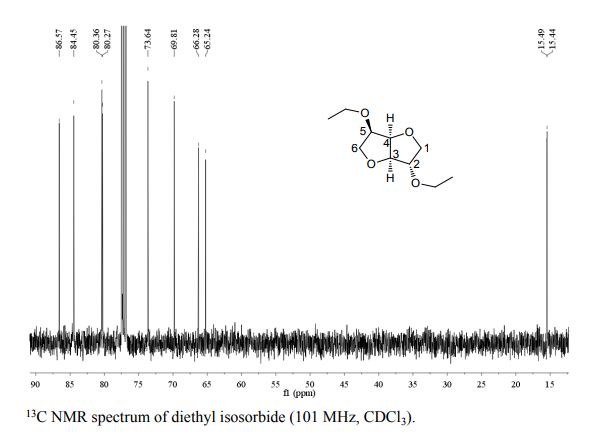

 Org. Biomol. Chem., 2017, Advance Article
Org. Biomol. Chem., 2017, Advance Article