
 Org. Biomol. Chem., 2017, Advance Article
Org. Biomol. Chem., 2017, Advance Article
DOI: 10.1039/C7OB02204B, Paper
Maliha Uroos, Phillip Pitt, Laurence M. Harwood, William Lewis, Alexander J. Blake, Christopher J. Hayes
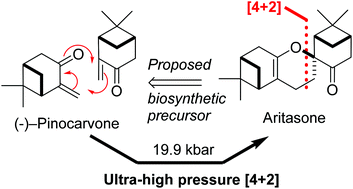
The total synthesis of aritasone via the proposed biosyntheic hetero-Diels-Alder [4 + 2] cyclodimerisation of pinocarvove, has been achieved under ultra-high pressure (19.9 kbar) conditions
Total synthesis of (−)-aritasone via the ultra-high pressure hetero-Diels–Alder dimerisation of (−)-pinocarvone
Author affiliations
*Corresponding authors
aSchool of Chemistry, University of Nottingham, University Park, Nottingham, UK
E-mail:chris.hayes@nottingham.ac.uk
Fax: +44 (0)115951 3564
Tel: +44 (0)115 951 3045
bChemical Sciences Division, University of Reading, Whiteknights, Reading, UK

Christopher Hayes
Abstract
This paper describes a total synthesis of the terpene-derived natural product aritasone via the hetero-Diels–Alder [4 + 2] cyclodimerisation of pinocarvove, which represents the proposed biosyntheic route. The hetero-Diels–Alder dimerisation of pinocarvone did not proceed under standard conditions, and ultra-high pressure (19.9 kbar) was required. As it seems unlikely that these ultra-high pressures are accessible within a plant cell, we suggest that the original biosynthetic hypothesis be reconsidered, and alternatives are discussed.
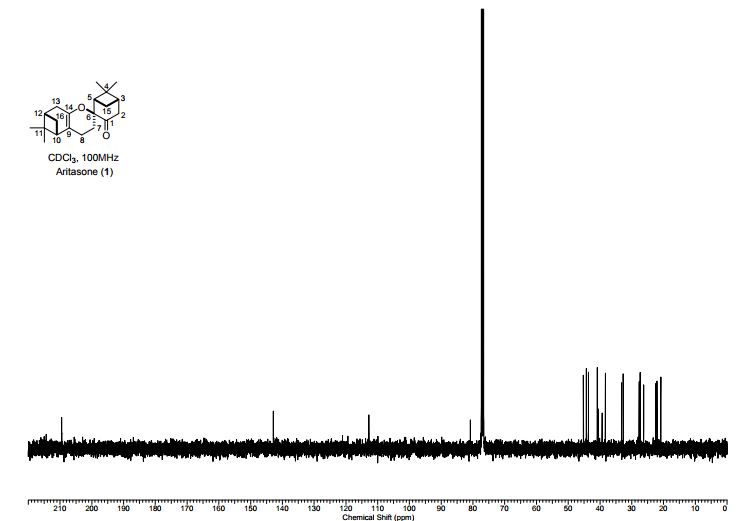
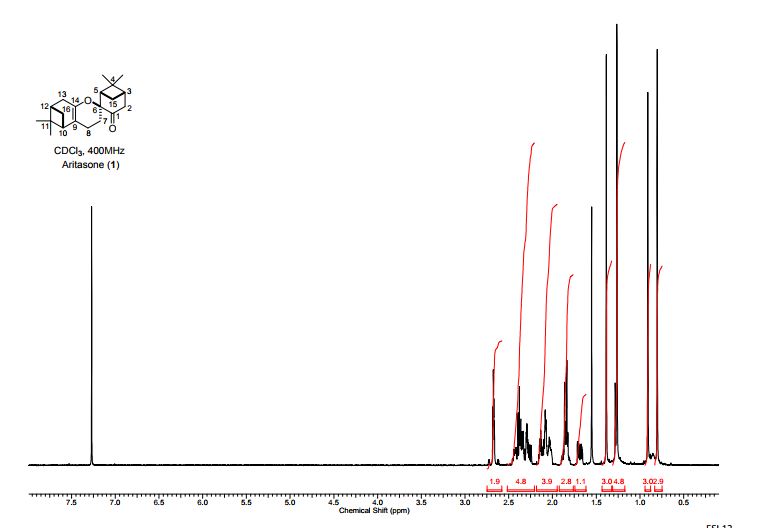
Aritasone (1) A solution of pinocarvone (()-2) (100 mg, 0.66 mmol) in dichloromethane (5 mL) was pressurized to 19.9 kbar for 120 h. The 1H NMR spectrum of the crude reaction mixture showed significant change in the composition as compared to the starting material. The solvent was evaporated and the residue was purified by column chromatography (pentane/Et2O; 25/1) to afford aritasone (1) (20 mg, 40%) as a white solid; mp 101- 103 C; (lit3 mp 105-106 °C); []D 26 26.1 (c 0.40 in CHCl3); (lit3 []D 9 118); max/cm-1 (CHCl3) 2926, 2359, 1722, 1689, 1601, 1467, 1372, 1305, 1152; H (400 MHz; CDCl3, 298 K) 2.67 (2H, app dd, J 4.8, 2.5, H-2a, H-2b), 2.45-2.32 (3H, m, H-7a, H-15a, H-3), 2.15-2.01 (4H, m, H-10, H-12, H-15b, H-16a), 1.91-1.80 (2H, m, H-4, H-16b), 1.66 (1H, ddd, J 13.8, 6.4, 3.4, H-7b), 1.38 (3H, s, CH3), 1.29-1.22 (7H, br s, CH3, H-13a, H-13b, H-8a, H- 8b), 0.90 (3H, s, CH3), 0.80 (3H, s, CH3); C (100 MHz; CDCl3, 298 K) 209.5 (C), 142.9 (C), 112.8 (C), 80.8 (C), 45.2 (CH), 44.3 (CH), 43.7 (CH2), 40.9 (CH), 40.5 (C), 39.4 (CH), 38.3 (C), 33.2 (CH2), 32.7 (CH2), 27.7 (CH3), 27.3 (CH2), 27.3 (CH3), 26.3 (CH3), 22.5 (CH2), 22.1 (CH2), 20.9 (CH3); HRMS m/z (ES+ ) found 301.2162 (M + H) C20H29O2 requires 301.2162 and 323.1981 (M + Na) C20H28O2Na requires 323.1982. These data were consistent to those previously reported, 5, 7 however the value of the specific rotation5 differs significantly from that measured during the original isolation work.3

Christopher Hayes
Contact
Biography
Prof. Christopher Hayes began his academic career here in Nottingham with his B.Sc. in July 1992. Remaining at Nottingham, he completed his Ph.D. studies in organic chemistry, under the supervision of Professor Gerald Pattenden, in September 1995. In January 1996, on a NATO Postdoctoral Fellowship, he moved to the University of California at Berkeley where he worked in the group of Professor Clayton H. Heathcock. In September 1997, he returned to Nottingham as a Lecturer in Organic Chemistry, and has subsequently been promoted to Reader (2003), Associate Professor (2006) and Professor of Organic Chemistry (2011).
Research Summary
Research is centred in main-stream synthetic organic chemistry, focusing on the organic chemistry of biologically active molecules. His current research interests span a number of areas such as (i)… read more
Recent Publications
-
-
BARTON, NAOMI A., MARSH, BENJAMIN J., LEWIS, WILLIAM, NARRAIDOO, NATHALIE, SEYMOUR, GRAHAM B., FRAY, RUPERT and HAYES, CHRISTOPHER J., 2016. Accessing low-oxidation state taxanes: is taxadiene-4(5)-epoxide on the taxol biosynthetic pathway?: Chemical Science Chemical Science. 7(5), 3102-3107
-
PALFRAMAN, MATTHEW J., ALHARTHY, RIMA D., POWALOWSKA, PAULINA K. and HAYES, CHRISTOPHER J., 2016. Synthesis of triazole-linked morpholino oligonucleotides via Cu-I catalysed cycloaddition: Organic & Biomolecular Chemistry Organic & Biomolecular Chemistry. 14(11), 3112-3119
-






















 Org. Biomol. Chem., 2017, Advance Article
Org. Biomol. Chem., 2017, Advance Article

