
The plant hormone methyl jasmonate can be used to increase broccoli’s antitumoral properties
http://www.chemistryviews.org/details/news/5428251/Boosting_Broccoli_Power.html

back to home for more updates
![]()

DR ANTHONY MELVIN CRASTO Ph.D

The plant hormone methyl jasmonate can be used to increase broccoli’s antitumoral properties
http://www.chemistryviews.org/details/news/5428251/Boosting_Broccoli_Power.html

back to home for more updates
![]()

DR ANTHONY MELVIN CRASTO Ph.D

The bark of Nauclea latifolia contains tramadol at medicinal concentrations © imagebroker / Alamy
http://www.rsc.org/chemistryworld/2013/09/african-plant-natural-source-tramadol
In another example of nature beating chemists, the African plant Nauclea latifolia has been found to be a natural source of the synthetic opioid tramadol. First marketed in 1977, tramadol is frequently used to relive moderate to moderately-severe pain. While other synthetic drugs have later been found in nature, this is the first instance where the discovery involves clinically viable concentrations.
Colloquially known as the ‘African peach’ or ‘pin cushion tree’, N. latifolia is a flowering, sub-Saharan evergreen that grows widely across Central and West Africa and is used by local populations to treat a wide variety of ailments – including epilepsy, malaria, general pain and many infectious diseases………………………. READ ALL AT
http://www.rsc.org/chemistryworld/2013/09/african-plant-natural-source-tramadol
tramadol

tramadol hydrocloride
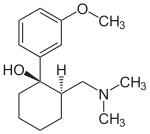
The chemical name for tramadol hydrochloride is (±)cis-2-[(dimethylamino)methyl]-1-(3methoxyphenyl) cyclohexanol hydrochloride
Tramadol (marketed as the hydrochloride salt by Janssen Pharmaceutica as Ultram in the United States, Ralivia by Biovail in Canada and many other companies throughout the world) is a centrally acting synthetic opioid analgesic used to treat moderate to moderately severe pain. The drug has a wide range of applications, including treatment of rheumatoid arthritis, restless legs syndrome, motor neurone disease and fibromyalgia.[citation needed] It was launched and marketed as Tramal by the German pharmaceutical company Grünenthal GmbH in 1977.
Tramadol is a weak μ-opioid receptor agonist, a serotonin releaser and a reuptake inhibitor of norepinephrine. Tramadol is metabolized to O-desmethyltramadol, a significantly more potent μ-opioid agonist. Tramadol and its major metabolite(s) are distinguished from other more potent opioid agonists by relative selectivity for μ-opioid receptors.
Structurally, tramadol closely resembles a stripped down version of codeine. Both codeine and tramadol share the 3-methyl ether group, and both compounds are metabolized along the same hepatic pathway and mechanism to the stronger opioid, phenol agonist analogs. For codeine, this is morphine, and for tramadol, it is the O-desmethyltramadol.
When administered through IV, patients notice very little clinical difference in subjective potency compared to morphine.
Structurally, tapentadol is the closest chemical relative of tramadol in clinical use. Tapentadol is also an opioid, but unlike both tramadol and venlafaxine, tapentadol represents only one stereoisomer and is the weaker of the two, in terms of opioid effect. Both tramadol and venlafaxine are racemic mixtures. Structurally, tapentadol also differs from tramadol in being a phenol, and not an ether. Also, both tramadol and venlafaxine incorporate a cyclohexyl moiety, attached directly to the aromatic, while tapentadol lacks this feature.

(1R,2R)-Tramadol (1S,2S)-Tramadol


(1R,2S)-Tramadol (1S,2R)-Tramadol
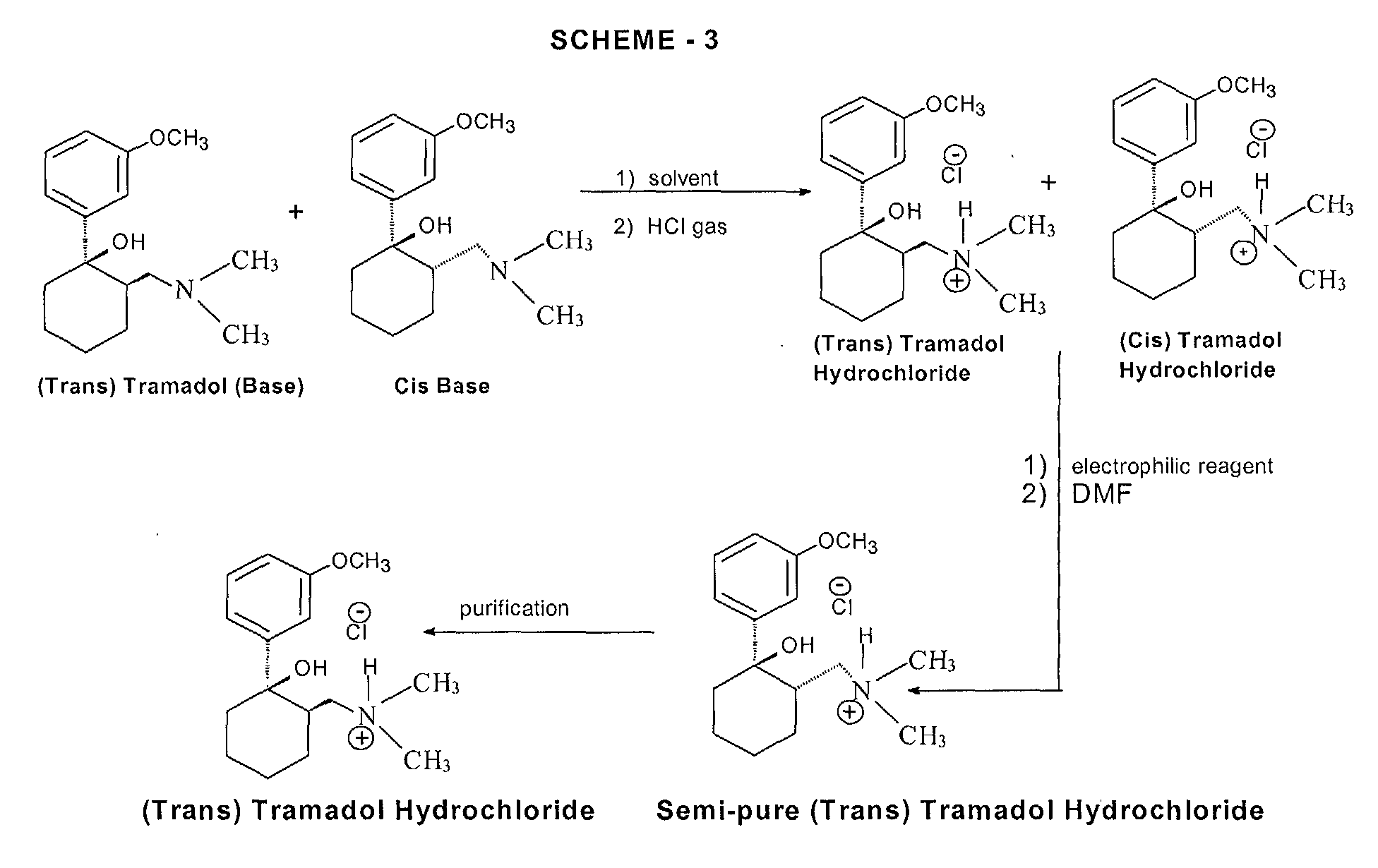
The chemical synthesis of tramadol is described in the literature.[62] Tramadol [2-(dimethylaminomethyl)-1-(3-methoxyphenyl)cyclohexanol] has two stereogenic centers at the cyclohexane ring. Thus, 2-(dimethylaminomethyl)-1-(3-methoxyphenyl)cyclohexanol may exist in four different configurational forms:
The synthetic pathway leads to the racemate (1:1 mixture) of (1R,2R)-isomer and the (1S,2S)-isomer as the main products. Minor amounts of the racemic mixture of the (1R,2S)-isomer and the (1S,2R)-isomer are formed as well. The isolation of the (1R,2R)-isomer and the (1S,2S)-isomer from the diastereomeric minor racemate [(1R,2S)-isomer and (1S,2R)-isomer] is realized by the recrystallization of the hydrochlorides. The drug tramadol is a racemate of the hydrochlorides of the (1R,2R)-(+)- and the (1S,2S)-(–)-enantiomers. The resolution of the racemate [(1R,2R)-(+)-isomer / (1S,2S)-(–)-isomer] was described[63] employing (R)-(–)- or (S)-(+)-mandelic acid. This process does not find industrial application, since tramadol is used as a racemate, despite known different physiological effects[64] of the (1R,2R)- and (1S,2S)-isomers, because the racemate showed higher analgesic activity than either enantiomer in animals[65] and in humans.[66]



Many of us have a sluggish metabolism. This can make it really difficult to lose weight and extremely easy to gain weight. A slow metabolism can also make you tire out easily so you don’t get to enjoy all that life has to offer. However, there is good news. You can easily boost your metabolism naturally if you know how. Below, you will find 7 ways to increase your metabolism:
read all at
http://www.fitnea.com/7-ways-of-boosting-your-metabolism/
highlight
Eat More Spicy Foods – Hot spices like curry, cayenne pepper, black pepper, cumin, and turmeric all help the body speed up your metabolism. There are other “hot” spices that you may not think of as hot but they react in the body in this way. They include cinnamon, cardamon, ginger, and nutmeg. Try adding these tasty spices to your soups, stir-fries, casseroles, and other dishes. Curry goes great in some type of salads like quinoa salads. Some people love the taste of cinnamon in their coffee. Just add it to the grounds before you brew it.

Helps support normal blood sugar levels with compounds called charantin and momordicin. Additional key compounds such as vicine, peptides, and polypeptide-p (plant insulin) also work together to give Bitter Melon its potency.
Bitter Melon (Momordica charantia Linn.)(fruit) Dried water extract).
read all at

Fish oils may take the brakes off the detrimental effects of some of the processes triggered in the brain by high-fat diets
read all at
http://newdrugapprovals.wordpress.com/2013/05/23/fish-oil-may-stall-effects-of-junk-food-on-brain/

Moringa oleifera
The Drumstick Plant
08 February 2013, Organic India, a manufacturer of herb-based functional supplements, has launched organic single ingredient Moringa products in the US.
Available in both capsule and powder formulations, the product made from powdered leaves of Moringa oleifera tree contains vitamin A, B1, B3, B12, iron, magnesium, potassium, amino acids, and polyphenols and is used for restoring internal imbalances.
Organic India national sales manager Heather Henning said the ancient therapeutic Moringa oleifera plant has been used for years and has seen increasing popularity amongst mainstream consumers worldwide.
Moringa oleifera leaf powder
“Millions of people globally use Moringa for essential nutrition — now, the US distribution channel will have access to this extraordinary plant with USDA organic certification,” Henning added.
The company said Moringa supplement, which has more B12 than steak, more vitamin A than eggs, and more calcium than milk, will be unveiled to the public at Expo West 2013.
Sonjna (Moringa oleifera) leaves with flowers
Moringa oleifera (synonym: Moringa pterygosperma) is the most widely cultivated species of the genus Moringa, which is the only genus in the family Moringaceae. English common names include moringa, and drumstick tree, from the appearance of the long, slender, triangular seed pods, horseradish tree, from the taste of the roots which resembles horseradish, or ben oil tree, from the oil derived from the seeds. The tree itself is rather slender, with drooping branches that grow to approximately 10m in height. In cultivation, it is often cut back annually to 1–2 meters and allowed to regrow so the pods and leaves remain within arm’s reach.[1][2]
In developing countries, moringa has potential to improve nutrition, boost food security, foster rural development, and support sustainable landcare.[3] It may be used as forage forlivestock, a micronutrient liquid, a natural anthelmintic and possible adjuvant.[2][4][5]

The moringa tree is grown mainly in semiarid, tropical, and subtropical areas, corresponding in the United States to USDA hardiness zones 9 and 10. While it grows best in dry, sandy soil, it tolerates poor soil, including coastal areas. It is a fast-growing, drought-resistant tree that is native to the southern foothills of the Himalayas in northwestern India.
Cultivation in Hawai’i, for commercial distribution in the United States, is in its early stages.[6]
“India is the largest producer of moringa, with an annual production of 1.1 to 1.3 million tonnes of tender fruits from an area of 380 km². Among the states, Andhra Pradesh leads in both area and production (156.65 km²) followed by Karnataka (102.8 km²) and Tamil Nadu(74.08 km²). In other states, it occupies an area of 46.13 km². Tamil Nadu is the pioneering state in·so·much as it has varied genotypes from diversified geographical areas and introductions from Sri Lanka.”[7]
Moringa is grown in home gardens and as living fences in Tamil Nadu Southern India and Thailand, where it is commonly sold in local markets.[8] In the Philippines, it is commonly grown for its leaves, which are used in soup.[9] Moringa is also actively cultivated by theWorld Vegetable Center in Taiwan, a center for vegetable research with a mission to reduce poverty and malnutrition in developing countries through improved production and consumption of vegetables. Tamil Nadu Southern India has Moringa in its folk stories and as well considered to be auspicious to grow in home. Interestingly the name in Tamil is Moorungai which sounds same as Moringa.
It is also widely cultivated in Africa, Cambodia, Nepal, Indonesia, Malaysia, Mexico, Central and South America, and Sri Lanka
An Indian drumstick (cut)
| Nutritional value per 100 g (3.5 oz) | |
|---|---|
| Energy | 64 kcal (270 kJ) |
| Carbohydrates | 8.28 g |
| – Dietary fiber | 2.0 g |
| Fat | 1.40 g |
| Protein | 9.40 g |
| Water | 78.66 g |
| Vitamin A equiv. | 378 μg (47%) |
| Thiamine (vit. B1) | 0.257 mg (22%) |
| Riboflavin (vit. B2) | 0.660 mg (55%) |
| Niacin (vit. B3) | 2.220 mg (15%) |
| Pantothenic acid (B5) | 0.125 mg (3%) |
| Vitamin B6 | 1.200 mg (92%) |
| Folate (vit. B9) | 40 μg (10%) |
| Vitamin C | 51.7 mg (62%) |
| Calcium | 185 mg (19%) |
| Iron | 4.00 mg (31%) |
| Magnesium | 147 mg (41%) |
| Manganese | 0.36 mg (17%) |
| Phosphorus | 112 mg (16%) |
| Potassium | 337 mg (7%) |
| Sodium | 9 mg (1%) |
| Zinc | 0.6 mg (6%) |
| Percentages are relative to US recommendations for adults. Source: USDA Nutrient Database |
|
| Nutritional value per 100 g (3.5 oz) | |
|---|---|
| Energy | 37 kcal (150 kJ) |
| Carbohydrates | 8.53 g |
| – Dietary fiber | 3.2 g |
| Fat | 0.20 g |
| Protein | 2.10 g |
| Water | 88.20 g |
| Vitamin A equiv. | 4 μg (1%) |
| Thiamine (vit. B1) | 0.0530 mg (5%) |
| Riboflavin (vit. B2) | 0.074 mg (6%) |
| Niacin (vit. B3) | 0.620 mg (4%) |
| Pantothenic acid (B5) | 0.794 mg (16%) |
| Vitamin B6 | 0.120 mg (9%) |
| Folate (vit. B9) | 44 μg (11%) |
| Vitamin C | 141.0 mg (170%) |
| Calcium | 30 mg (3%) |
| Iron | 0.36 mg (3%) |
| Magnesium | 45 mg (13%) |
| Manganese | 0.259 mg (12%) |
| Phosphorus | 50 mg (7%) |
| Potassium | 461 mg (10%) |
| Sodium | 42 mg (3%) |
| Zinc | 0.45 mg (5%) |
| Percentages are relative to US recommendations for adults. Source: USDA Nutrient Database
|
|

BITTER MELON (Momordica charantia): This edible gourd should be every physician’s “go-to” plant for the 16 million or more Americans with high-normal glucose readings or ‘boderline diabetic/metabolic syndrome patients.
Preliminary evidence suggests bitter melon’s hypoglycemic action can be explained through several independent mechanisms: for one, it has been shown to increase peripheral glucose oxidation as well as glucose tolerance and insulin signaling in induced insulin resistance models (Sridhar MG, et al: Br J Nutr. 2008;99(4):806-12. Basch E, et al. Am J Health Syst Pharm. 2003;60:356-9). It also decreases hepatic gluconeogenesis, while increasing glycogen synthesis.
Bitter Melon increases insulin output from the pancreas, and it also provides a unique compound called polypeptide-P, which is an insulin mimetic with a similar structure to bovine insulin (Krawinkel MB, Keding GB. Nutr Rev. 2006;64(7 Pt 1):331-7).
 Bitter Melon slices.
Bitter Melon slices.Compounds produced by this intriguing gourd have been shown to reduce triglyceride levels in a dose-dependent manner in animal trials (Jayasooriya AP, et al. J Ethnopharmacol. 2000;72:331-6). Though we don’t yet have human data corroborating this effect, the animal studies suggest that bitter melon may have a role in reducing cardiovascular risk, particularly in people with diabetes or metabolic syndrome.
Bitter melon products are typically standardized to their constituents, momordicosides and charantin, and usually dispensed in 500-600 mg doses, twice daily, following meals. As it does have an insulin mimetic action, it may be necessary to adjust the dose of concurrently prescribed hypoglycemic drugs.