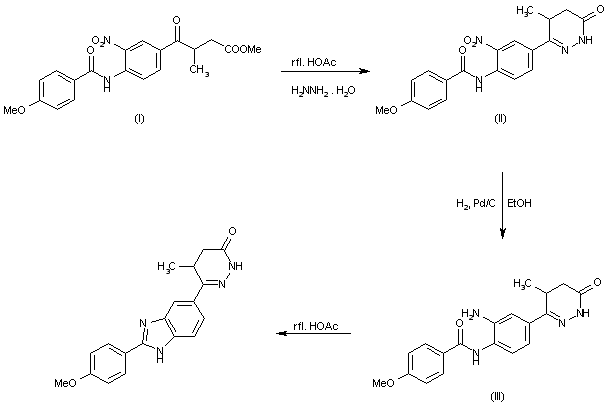
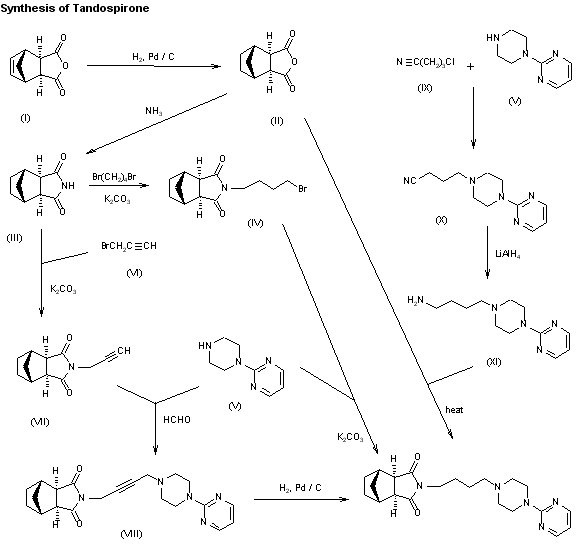
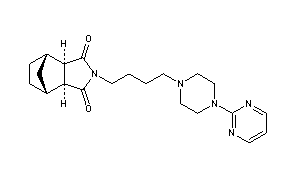
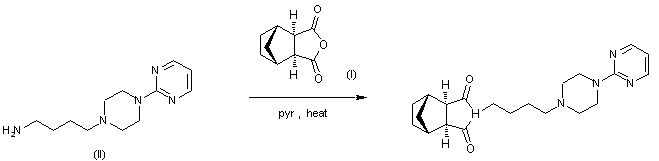TRANDOLAPRIL
(2S,3aR,7aS)-1-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-octahydro-1H-indole-2-carboxylic acid
87679-37-6 CAS NO
C24-H34-N2-O5, 430.549
Indications. hypertention
Abbott..(opten , godrik, mavik), HOECHST MARION ROUSSEL..Odrik,
RU-44570, Preran,
Aventis Pharma (Originator), Nippon Roussel (Originator), Abbott (Licensee), Chugai (Licensee) Launched-1993
Launched-1993
Trandolapril is a non-sulhydryl prodrug that belongs to the angiotensin-converting enzyme (ACE) inhibitor class of medications. It is metabolized to its biologically active diacid form, trandolaprilat, in the liver. Trandolaprilat inhibits ACE, the enzyme responsible for the conversion of angiotensin I (ATI) to angiotensin II (ATII). ATII regulates blood pressure and is a key component of the renin-angiotensin-aldosterone system (RAAS). Trandolapril may be used to treat mild to moderate hypertension, to improve survival following myocardial infarction in clinically stable patients with left ventricular dysfunction, as an adjunct treatment for congestive heart failure, and to slow the rate of progression of renal disease in hypertensive individuals with diabetes mellitus and microalbuminuria or overt nephropathy.
Trandolapril is an ACE inhibitor used to treat high blood pressure, it may also be used to treat other conditions. It is marketed by Abbott Laboratories with the brand name Mavik.
Tarka is the brand name of an oral antihypertensive medication that combines a slow release formulation of verapamil hydrochloride, acalcium channel blocker, and an immediate release formulation of trandolapril, an ACE inhibtor. The patent, held by Abbott Laboratories, expires on February 24, 2015.
This combination medication contains angiotensin-converting enzyme (ACE) inhibitor and calcium channel blocker, prescribed for high blood pressure.

Trandolapril is a prodrug that is deesterified to trandolaprilat. It is believed to exert its antihypertensive effect through the renin-angiotensin-aldosterone system. Trandolapril has a half life of about 6 hours, and trandolaprilat has a half life of about 10. Trandolaprilat has about 8 times the activity of its parent drug. Approximately 1/3 of Trandolapril and its metabolites are excreted in the urine, and about 2/3 of trandolapril and its metabolites are excreted in the feces. Serum protein binding of trandolapril is about 80%.
Trandolapril is a drug that is used to lower blood pressure. Blood pressure is dependent on the degree of constriction (narrowing) of the arteries and veins. The narrower the arteries and veins, the higher the blood pressure. Angiotensin Il is a chemical substance made in the body that causes the muscles in the walls of arteries and veins to contract, narrowing the arteries and veins and thereby elevating blood pressure. Angiotensin Il is formed by an enzyme called angiotensin converting enzyme (ACE). Trandolapril is an inhibitor of ACE and blocks the formation of angiotensin Il thereby lowering blood pressure. The drop in blood pressure also means that the heart does not have to work as hard because the pressure it must pump blood against is less. The efficiency of a failing heart improves, and the output of blood from the heart increases. Thus, ACE inhibitors such as trandolapril are useful in treating heart failure.
Trandolapril‘s ACE-inhibiting activity is primarily due to its diacid metabolite, trandolaprilat, which is approximately eight times more active as an inhibitor of ACE activity.
……………………
synthesis

(3aR,7aS)-octahydroindole-2(S)-carboxylic acid (I) goes through the process of esterification with benzyl alcohol (II) in the presence of SOCl2 to produce the corresponding benzyl ester (III), and the yielding compound is then condensed with N-[1(S)-(ethoxycarbonyl)-3-phenylpropyl]-(S)-alanine (IV) in the presence of 1-hydroxybenzotriazole, N-ethylmorpholine and dicyclohexylcarbodiimide (DCC) in DMF to afford the benzyl ester (V) of the desired product. Lastly, the compound is debenzylated by hydrogenation with H2 over Pd/C in ethanol.
……………………………………………..
Trandolapril along with other related compounds was first disclosed in US4933361. The process for the synthesis of trandolapril was described in US4933361 and WO9633984.
US4933361 describes a process for the synthesis of trandolapril wherein the racemic benzyl ester of octahydro indole-2-carboxylic acid is reacted with N-[1-(S)-ethoxy carbonyl- 3- phenyl propyl]-L-alanine (ECPPA), to get racemic benzyl trandolapril, which is purified using column chromatography to get the 2S isomer of benzyl trandolapril, which is further debenzylated with Pd on carbon to get trandolapril as a foamy solid. This process has certain disadvantages, for example the product is obtained in very low yield. Purification is done using column chromatography, which is not suitable for industrial scale up.
WO9633984 discloses a process in which N-[1-(S)-ethoxy carbonyl-3- phenyl propyl]-L- alanine is activated with N-chlorosulfinyl imidazole, to get (N-[I-(S) N-[1-(S)-ethoxy carbonyl-3-phenyl propylj-L-alanyl-N-sulfonyl anhydride and which is further reacted with silyl-protected 2S,3aR,7aS octahydro indole 2-carboxyIic acid to obtain trandolapril. The main disadvantages of this process are that the silyl-protected intermediates are very sensitive to moisture, the process requires anhydrous conditions to be maintained and the solvent used has to be completely dried. It is very difficult to maintain such conditions on an industrial scale, and failing to do so leads to low yield of product.
The processes for preparing N-[1-(S)-ethoxy carbonyl-3-phenyl propyl]-L-alanine N- carboxyanhydride which is used in the process of the present invention are well known and are disclosed in JP57175152A, US4496541 , EP215335, US5359086 and EP1197490B1. Trans octahydro-IH-indole-2-carboxylic acid and its esters are the key intermediates in the synthesis of trandolapril. When synthesized, trans octahydro-1 H-indole-2-carboxylic acid is a mixture of four isomers, as shown below.

From the processes known in the prior art, trans octahydro-1 H-indole-2-carboxylic acid is converted to its ester and the ester is then either reacted directly with N-[1-(S)-ethoxy carbonyl-3-phenyl propyl]-L-alanine (ECPPA) and then the isomers are separated by column chromatography, or alternatively the ester is reacted with ECPPA followed by 0 deprotection. Trans octahydro-1 H-indole-2-carboxylic acid is always used in its protected form. No attempts have been made to resolve free trans octahydro-1 H-indole-2-carboxylic acid to convert it to the desired isomer (isomer D, above). Furthermore, none of the prior art processes is stereoselective, so resolution of the required isomer is required following condensation.
EP0088341 and US4490386 describe a method for the resolution of N-benzoyl (2RS,3aR,7aS) octahydro-1 H-indole-2-carboxylic acid using α-phenyl ethyl amine.
US6559318 and EP1140826 describe a process for the synthesis of (2S,3aR,7aS) 0 octahydro-1 H-indole-2-carboxylic acid using enzymatic resolution of its nitrile intermediate. Enzymatic resolution involves many steps and also requires column chromatography for purification making the process uneconomical industrially.
WO8601803 describes the preparation of (2S,3aR,7aS) octahydro-1 H-indole-2-carboxylic 5 acid ethyl ester and benzyl ester using 10-D-camphor sulphonic acid.
WO2004065368 describes the synthesis of (2S,3aR,7aS) octahydro-1 H-indole-2- carboxylic acid benzyl ester by resolution using 10-D-camphor sulphonic acid to prepare trandolapril. This process gives poor yields because the product has to be first resolved and then the ester is deprotected leading to further loss in yield, making the process low yielding and expensive.
W 02005/051909 describes a process for the preparation of trandolapril, i.e. (N-[I-(S)- carbethoxy-3-phenylpropyl}-S-alanyl-2S,3aR,7aS-octahydroindol-2-carboxyIic acid} as well as its pharmaceutical acceptable salts, using a racemic mixture of trans octahydroindole-2- carboxylic acid with the N-carboxyanhydride of {N-[1-(S)-ethoxycarbonyl-3-phenylpropyl}- S-alanyl (NCA) in a molar ratio of 1 :1 to 1.6:1 in a mixture of water and water-miscible solvent to obtain a mixture of diastereomers of trandolapril. The diastereomers are converted to salts which upon repeated crystallization from acetone and water, and reaction with a base gives pure trandolapril. Thus, the condensation reaction in the presence of water and a water-miscible solvent is not stereoselective.
The processes for preparing N-[1-(S)-ethoxy carbonyl-3- phenyl propyl]-l_-alanine N- carboxyanhydride starting from N-[1-(S)-ethoxy carbonyl-3- phenyl propyl]-L-alanine (ECPPA) are well known and are disclosed in JP57175152A, US4496541 , EP215335, US5359086 and EP1197490B1
The angiotensin-converting enzyme (ACE) inhibitor trandolapril is commonly prescribed as a cardiovascular drug for the control and management of mild to severe hypertension Chigh blood pressure) and may be used alone or in combination with diuretics or other antihypertensive agents. Administration of trandolapril is typically oral at a level of around 0.5-4 mg once a 15 day and may also be used in the management of conditions such as heart failure and left ventricular dysfunction following myocardial infarction.
Trandolapril itself is a prodrug, being converted to the acid form “trandolaprilat” in vivo. It is, however, • generally desirable to prepare and administer the ester form.. The structures of trandolapril and trandolaprilat ‘ are shown below.
Trandolapril Trandolaprilat
Various methods for the synthesis of trandolapril and related compounds have been proposed but each of these suffers from drawbacks . Frequently the syntheses require the use of dangerous reagents, which make industrial scale preparation hazardous and difficult and/or involve multiple steps resulting in a long and complex synthesis . One of the most important steps in the synthesis is the formation of the trans-fused octahydroindole ring, which is often difficult to separate from the cis-fused equivalent.
A number of the known synthetic routes to trandolapril proceed via the key intermediate (2S, 3aR,7aS) -octahydro,-lH-indole-2-carboxylic acid. This contains the key trans-fused octahydroindole ring and the correct stereochemistry for the carboxylic acid group at the 2-position. Frequently, these methods require the separation of the cis- and trans-fused rings and, in many cases, resolution of the carboxylate group at the 2 -position is necessary. Where production of the trans-fused ring junction has been possible without generating significant quantities of the cis-product, the syntheses have been long and/or required dangerous reagents such as mercury compounds.
(2S, 3aR, 7aS)-octahydro-lH-indole-2-carboxylic acid
US-A-4691022 gives a synthesis of the above intermediate compound in relatively few steps but requires the trans-octahydroindole as the starting material. The result is also a mixture of the 2-α and 2-β compounds.
EP-A-084164/US-A-4, 933,361 provides an apparently effective method for the synthesis of the cis-fused intermediate beginning with the high-pressure hydrogenation of indole at 100 atmospheres of hydrogen and a platinum catalyst. This document also provides two methods for forming the trans-fused octahydroindole ring, but neither is indicated as being efficient. The first method provides the stereochemistry for the 2 -position from substituted alanine, reacting this with activated cyclohexanone and cyclising the product to give a hexahydroindole . Unfortunately, the reduction of this hexahydroindole to the octahydro- compound produces both cis- and trans-fused product in unknown yield. The second method is to introduce the trans-ring via trans-octahydro-lH-quinolin-2 -one, but no indication of yield in the key step is given and complex series of halogenation, partial re-hydrogenation and re-arrangement are required to reach the desired intermediate .
WO 00/40555 / US 6559318 relies on enzymic resolution of a 2- (2 ‘ , 2 ‘ -methoxyethyl) cyclohexamine with Novozyme7 over 25 hours to provide the N-acetylated (1R, 2S) enantiomer which must then be separated by column chromatography from the. unreacted (IS, 2R) enantiomer. Neither the enzymic resolution nor the chromatography steps are well suited to industrial scale preparations. There are also around ten steps required to reach the desired compound.
The synthetic route to the above octahydroindole intermediate proposed by Henning et al . (Tett. Lett. 24(1983), 5343-5346) quickly and elegantly introduces a 1,2-trans configuration around a cyclohexane ring, but requires the use of mercuric nitrate. The use of mercury compounds is obviously undesirable in the preparation of pharmaceuticals. A further synthesis is provided by Brion et al . (Tett. Lett. 33 (1992) 4889-4892) but it is unclear whether they in fact prepare 5% or 95% of the desired product with 2S stereochemistry. In any case, the method requires eleven steps including an initial pig liver esterase digestion to provide the product in stereochemically pure form but in a 95:5 mixture of isomers at position 2. This method is thus complex and ill suited to industrial scale preparation.
ROUTE A – Separation of enantiomers by the formation of diastereomeric salts with a chiral resolving agent HA* (such as 0, O’ -dibenzoyl-L-tartaric acid), coupling with N- [1- (S) -ethoxycarbonyl-3-phenylpropyl] -L-alanine (ECPPA) derivative and finally deprotecting the carboxylic acid moiety Rλ (such as by hydrogenating a benzyl ester, where Rx = Bn) .
ROUTE B.- Direct reaction of 7A with ECPPA derivative that leads to the formation of diastereoisomers, deprotecting the carboxylic acid moiety and finally separation of diastereoisomers by conventional methods.
1) deprotection >■ trandolapril 2) separation of diastereoisomers
ROUTE C- Treatment of 7A in basic medium and deprotection that leads to the racemic mixture of octahydroindole acid followed by the reaction with ECPPA derivative. This will result in a diastereomeric mixture that can be separated by conventional methods.
COOEtCH,
,1 ) basic medium QC &° ‘ – trandolapril 2) deprotection
2) separation of
racemic diastereoisomers 7A 6C
Route D. Separation of isomers of 6C by conventional methods (i.e. formation of a diastereomeric salt) and coupling with ECCPA derivative.
trandolapril
Route E
This route is an inversion of the steps of route B Firstly the isomers are separated and then the protecting group is removed. 1) separation of diastereoisomers trandolapπl
racemic 2) deprotection
Route F. – The compound 8A is treated to remove the protecting grqup and coupled with an ECPPA derivative,
1) deprotection
2) base treatment
racemic 7A 8A
X activating group
………………………………………………………………………………
US20060079698
http://www.google.com/patents/US20060079698

…………..
INTERMEDIATE
(2S,3aR,7aS)-perhydroindole-2-carboxylic acid (42 g).
IR (Nujol, cm-1): 2923, 2854, 1600, 1458, 1377, 1319. 1H-NMR (D2O): δ 1.1-2.5 (m, 8H), 1.65(m,1H), 1.96-2.37 (m,2H), 2.91(td, 1H),4.46(d, 1H). Mass (m/z): 168.3(M-H).
http://www.faqs.org/patents/app/20110065930
(2S,3aR,7aS)-Octahydro-1H-indole-2-carboxylic acid hydrochloride
yield as a white solid.
1H NMR (D2O, 400 MHz): δ 4.42 (dd, 1H, J=11.1, 2.7 Hz), 2.93, (dt, 1H, J=11.8, 3.6 Hz), 2.36 (ddd, 1H, J=12.9, 6.7, 2.7 Hz), 2.31-2.16 (m, 1H), 2.11-2.01 (m, 2H), 1.92-1.90 (m, 1H), 1.79-1.75 (m, 1H), 1.68-1.53 (m, 2H), 1.34-1.13 (m, 3H);
LC-MS (m/z): 170.1 (M+H).sup.+. The isolated product (5) correlates to the material prepared according to U.S. Pat. No. 487,932 and Tetrahedron Lett., 1992, 33, 4889.
(2s,3aR,7aS)-octahydro-1H-indole-2-carboxylic acid HCl
CAS No: 144540-75-0

……………………………………..
REF
Tan, X; He, W; Liu, Y (2009). “Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy”. Kidney international 76 (12): 1248–57. doi:10.1038/ki.2009.346. PMID 19759524.
Drugs Fut1989,14,(8):778
Urbach, H., Henning, R., Teetz, V., Geiger, R., Becker, R. and Gaul, H. (Hoechst A.G.) Bicyclic amino acid derivatives.DE 3151690, EP 084164, EP 170775.JP 1989301659; JP 1989301695
……………………………………………………………………….
The ESI mass spectrum of the drug trandolapril displayed a molecular ion peak [M+H] + at 431.1 amu. The tandem mass spectra (MS2) showed the fragment ions at m/z 234.2, 170.2, 160.3, 134.2, 130.3, 117.2, 102.3 and 91

The IR spectrum of new impurity showed the following absorption bands 3277cm-1 (NH stretch), 2941cm-1 (aliphatic CH stretch), 1734 and 1653cm-1 (C=O) stretch and 1192cm-1 (C-O stretch)

1H NMR
13 C NMR

TRP = TRANDOLAPRIL COMPARED WITH 2 IMPURITIES


………………………………………………………………………………………
TRANDOLAPRIL SPECTRAL DATA
http://www.google.com.br/patents/US20060079698
IR (KBr, cm-1): 3444, 3280, 2973, 2942, 2881, 1735, 1654, 1456, 1367, 1193, 1024, 699.
The 1H-NMR (CDCl3): δ 7.2 (s, 5H), 4.4(m,4H), 4.2 (q,2H), 3.6-1.3 (m, 18H), 1.28(d+t,6H). CI Mass (m/z): 429.6(M-H).
……………………………………
United States Patent Application 20080171885
http://www.freepatentsonline.com/y2008/0171885.html
M.P.: 122-124° C.,
IR (KBr): 3278.7, 2942.2, 1735.2, 1654.3, 1456.7, 1433.7, 1366.5, 1192.8, 1101.5, 1063.8 and 1023.8 cm−1 (FIG. 1).
1H NMR (CD3OD, δ ppm): 7.33 (s, 5H), 4.34 (m, 3H), 3.86 (q, 2H), 3.28-1.46 (m, 17H) and 1.39 (d+t, 6H),
Mass (m/z, amu): 453.5 (M+Na) and 431.7 (M+H)+ molecular ion.
…………………………………………………………………………….
MORE INFO FOR READERS
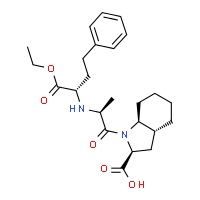
trandolapril
-
synthesis of organic compounds related to L-alanine, which are starting materials for synthesizing building blocks needed for the production of indole-like inhibitors of Angiotensin I Converting Enzyme (IACE), namely Trandolapril and its derivatives.
-
[0002]
More specifically the invention relates to a new synthesis of Trandolapril and other indole-like IACE, which are potent hypertension inhibitors.
-
[0003]
Trandolapril is a known antihypertensive agent defined as (2S, 3aR, 7aS)-1-[(1S)-1-ethoxycarbonyl)-3-phenylpropylamino-1-oxopropyl] octahydro-[1 H]-indole-2-carboxylic acid. Trandolapril has the following structural formula:
-
The general approach in most of the Trandolapril synthesis is a peptide coupling of N-[(1-ethoxy carbonyl)-3-phenyl propyl)-S-alanine with benzyl-(2s,3aR,7aS)-octahydroindole-2-carboxylate using as coupling agent dicyclohexylcarbodiimiide in combination with 1-hydroxy benzotriazole or n-alkyl phosphonic anhydride in presence of an organic base, such as triethylamine. (2S,3aR,7aS)-octahydroindole-2-carboxylic acid is a key intermediate for the synthesis of trandolapril, which is described in the
US Patent 4,525,803 .
-
[0005]
The synthesis of the key intermediate is described in the following patents or publications viz.,
Tetrahedron Letters, Vol. 24, (48), 5339-5345;
Tetrahedron Letters, Vol. 24, (48), 5347-5350 ;
US Patent 4.879.392 ; US Patent 49633361 / EP 084164 ;
Tetrahedron Letters Vol. 35 (54), 4889-4892; and
US Patent 6, 559, 318 .
-
[0006]
The synthesis of octahydroindole-2-carboxylic acid as described in
Tetrahedron Letters, Vol. 24, (48), 5339-5345 is given in the scheme-I
-
[0007]
In this method, trans decahydroquinoline derivative of formula-Xlla is subject to Favorskii type ring contraction, followed by hydrolysis to give a mixture of III a and III b as a 1:1 mixture.
-
[0008]
A similar reaction with
cis derivative XII b gives a mixture of IIIc and IIId as a 9:1 mixture.
-
[0009]
The selectivity for IIIc over IIId, when the reaction is conducted with cis lactam Xllb, is due to less thermal instability of IIIb on account of 1,3-cis interaction of a carboxyl group and a six-member ring. Such interaction, is not present in IIIa and IIIb, formed from trans lactam XIIa, hence the product is formed as a 1:1 mixture
The scheme-II describes the methodology used in
Tetrahedron Letters, Vol. 24, (48), 5347-5350 for the preparation of trans octahydroindole-2-carboxylic acid
Reaction of cyclohexene with acetonitrile and mercuric acetate followed by ligand exchange with sodium chloride gives the crystalline acetamidomercury chloride in 98% yield. Reaction of the product of formula XIIIa with α-chloro acrylonitrile followed by reaction with NaBH4 and ethanol gives the product of formula XIIIb, which is cyclized with sodium in DMF to get a mixture of Xlllc and XIIId in the ratio of 18.5 : 1. On hydrolysis, IIIa is obtained selectively.
-
[0010]
Another method of preparation for octahydroindole-2-carboxylic acid is disclosed in the
US Patent 4,879,392 , and is reported in scheme III
-
[0011]
Herein, the cyclohexane derivative of formula XIV is converted into octahydroindole-2-carbonitrile the derivative of formula XV, which is hydrolyzed to give octahydroindole-2-carboxylic acid of formula III a.
-
[0012]
Another method for the synthesis of octahydroindole-2-carboxylic acid and its subsequent conversion to trandolapril is disclosed in the
US Patent 4963361 / EP 084164 and given in the scheme IV
-
[0013]
In this patent, methyl-β-chloro alaninate hydrochloride of formula XVI is acetylated to give a product of formula XVII, which is treated with the enamine derivative of formula XVIII to give hexahydroindoline-2-carboxylicacid of formula-IV. The product of formula IV is hydrogenated and the required enantiomer is isolated by cooling to -20°C. (2S,3aR,7aS)-Octahydroindole-2-carboxylic acid is first esterified with benzyl alcohol, coupled with ECPPA using DCC/HoBT, and finally debenzylated to yield trandolapril.
Tetrahedron Letters Vol. 35 (54), 4889-4892 describes another methodology for the synthesis of (2S,3aR,7aS)-octahydroindole-2-carboxylic acid, which is depicted in scheme V
-
[0014]
Dimethyl-1,2-cyclohexane dicarboxylate of formula XX is enzymatically resolved to give the monomethyl ester of 1,2-cyclohexane dicarboxylic acid of formula XXI, which is converted into hexahydroisobenzofuranone of formula XXII. The product of formula XXII is reacted with pyrrolidine to yield a product of formula XXIII which is converted to hexahydroisobenzofuranone of formula XXII a. This product is treated with ammonia to give cyclohexane carboxamide of formula XXV. This product is subject to the Hoffmann reaction, followed by reaction with formaldehyde and potassium cyanide to give cyclohexyl amine derivative of formula XXVI. The product of formula XXVI, in reaction with methane sulphonyl chloride and benzoyl chloride give a product of formula XXVII. This product is converted into a mixture of octahydroindole-2-carbonitrile of formula XXVIII a and XXVIII b. Octahydrindole-2-carbonitrile is hydrolyzed to give octahydroindole-2-carboxylic acid of formula III a.
The process for the synthesis of (2S,3aR,7aS)-octahydroindole-2-carboxylic acid is described in the
US Patent 6,559,318 and reported in the scheme VI.
In this method, cyclohexylamine derivative of formula-XXIX is resolved to produce enantiomerically pure product of formula XXX, which is converted to octahydroindole-2-carbonitrile of formula XXVIII a. The product of formula XXVIII a on hydrolysis yields the octahydroindole-2-carboxylic of formula III a.
-
[0015]
The above description gives various methods adopted to synthesize octahydroindole-2-carboxylic acid, which is the key intermediate in the preparation of trandolapril. After analyzing the different methods, it can be concluded that except the methodologies described in the
US Patent 4963361 / EP 084164 , all the other methods are not suitable for industrial purpose.
-
[0016]
The method described in the
US Patent 4963361 / EP 084164 has also the following drawbacks:
- i) The synthesis of methyl -β-chloro alaninate makes use of phosphorous pentachloride, which is a corrosive reagent and difficult to handle.
- ii) Isolation of (2S,3aR,7aS)-octahydroindole-2-carboxylic acid at -20°C is a difficult attempt during the scale up
- iii) Use of dicyclohexylcarbodiimiide in combination with hydroxybenzotriazole makes the process costlier