
Motolimod
VTX-2337, 莫托莫德 , мотолимод , موتوليمود ,
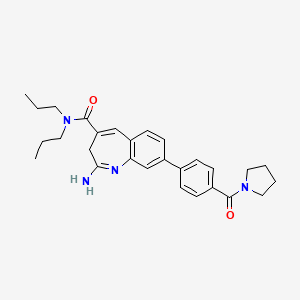
(1E,4E)-2-amino-N,N-dipropyl-8-(4-(pyrrolidine-1-carbonyl)phenyl)-3H-benzo[b]azepine-4-carboxamide,
- C28H34N4O2
- 458.595
Cancer; Lymphoma
George A. Doherty, C. Todd Eary, Robert D. Groneberg, Zachary Jones
| Originator: | Array BioPharma |
| Developer: | VentiRx Pharmaceuticals |
| Class: | Antineoplastics, immunomodulator |
| Mechanism of Action: | Toll-like receptor 8 (TLR8) agonist |
| WHO ATC code: | L03A-X |
| EPhMRA code: | L3A9 |
Useful for treating a toll-like receptor (TLR)-associated diseases eg cancer. VentiRx, under license from Array BioPharma, and collaborator Celgene are developing Motolimod
A TLR-8 agonist, for treating cancer. In June 2016, Motolimod was reported to be in phase 2 clinical development.
Clinical Trials:
| Conditions | Phases | Interventions | Recruitment |
| Epithelial Ovarian Cancer|Fallopian Tube Cancer|Primary Peritoneal Cancer | Phase 2 | Combination | Active, not recruiting |
| Carcinoma, Squamous Cell of Head and Neck | Phase 2 | Combination | Active, not recruiting |
| Ovarian Cancer | Phase 1|Phase 2 | Combination | Not yet recruiting |
| Low Grade B Cell Lymphoma | Phase 1|Phase 2 | Combination | Terminated |
| Locally Advanced, Recurrent, or Metastatic Squamous Cell Cancer of Head and Neck | Phase 1 | Combination | Completed |
| Recurrent or Persistent Ovarian Epithelial, Fallopian Tube, or Peritoneal Cavity Cancer | Phase 1 | Combination | Completed |
| Squamous Cell Carcinoma of the Head and Neck | Phase 1 | Combination | Recruiting |
| Advanced Solid Tumors|Lymphoma | Phase 1 | Alone | Completed |
CLICK TO VIEW
Biological Activity
| Description | Motolimod (VTX-2337) is a selective and potent Toll-like receptor (TLR) 8 agonist with EC50 of 100 nM, > 50-fold selectivity over TLR7. Phase 2. | |||||
|---|---|---|---|---|---|---|
| Targets | TLR8 [1] | |||||
| IC50 | 100 nM(EC50) | |||||
| In vitro | VTX-2337 stimulates the production of both TNFα with EC50 of 140 nM and IL-12 with EC50 of 120 nM in PBMCs. In monocytes and mDCs, VTX-2337 selectively induces the production of TNFα and IL-12 via NF-κB activation. VTX-2337 also stimulates IFNγ production from NK cells, augments the lytic function of NK cells and enhances ADCC. [1] | |||||
| In vivo | In an ovarian cancer mouse model, TX-2337 enhances the effect of pegylated liposomal doxorubicin (PLD). [2] | |||||
| Features | ||||||
Protocol(Only for Reference)
Kinase Assay: [1]
| Activity assay | The activity of specific TLR agonists is assessed using the secretory embryonic alkaline phosphatase (SEAP) reporter gene that is linked to NF-κB activation in response to TLR stimulation. Measurement of SEAP activity using the Quanti-blue substrate (InvivoGen) after TLR agonist treatment is carried out. |
|---|
Cell Assay: [1]
| Cell lines | PBMCs or purified NK cells |
|---|---|
| Concentrations | ~500 nM |
| Incubation Time | 48 h |
| Method | PBMCs or purified NK cells are prepared as previously described, and the purity of NK cells was approximately 99%. NK cell–mediated cytotoxicity is assessed by Calcein AM release from labeled target cells. In brief, PBMCs or purified NK cells are cultured for 48 hours in RPMI medium in the presence of VTX-2337 (167 or 500 nmol/L) before incubation with target cells. |
Conversion of different model animals based on BSA (Value based on data from FDA Draft Guidelines)
| Species | Mouse | Rat | Rabbit | Guinea pig | Hamster | Dog |
| Weight (kg) | 0.02 | 0.15 | 1.8 | 0.4 | 0.08 | 10 |
| Body Surface Area (m2) | 0.007 | 0.025 | 0.15 | 0.05 | 0.02 | 0.5 |
| Km factor | 3 | 6 | 12 | 8 | 5 | 20 |
| Animal A (mg/kg) = Animal B (mg/kg) multiplied by | Animal B Km |
| Animal A Km |
For example, to modify the dose of resveratrol used for a mouse (22.4 mg/kg) to a dose based on the BSA for a rat, multiply 22.4 mg/kg by the Km factor for a mouse and then divide by the Km factor for a rat. This calculation results in a rat equivalent dose for resveratrol of 11.2 mg/kg.
| Rat dose (mg/kg) = mouse dose (22.4 mg/kg) × | mouse Km(3) | = 11.2 mg/kg |
| rat Km(6) |
References
[1] Lu H, et al. Clin Cancer Res. 2012, 18(2), 499-509.
[2] Monk BJ, et al. J Clin Oncol 31, 2013 (suppl; abstr 3077).
Clinical Trial Information( data from http://clinicaltrials.gov, updated on 2016-06-25)
| NCT Number | Recruitment | Conditions | Sponsor /Collaborators |
Start Date | Phases |
|---|---|---|---|---|---|
| NCT02650635 | Recruiting | Colorectal Adenocarcinoma|Metastatic Pancreatic Adenocarcinoma|Recurrent Breast Carcinoma|Recurrent Colorectal Carcinoma|Recurrent Melanoma of the …more | Mayo Clinic|National Cancer Institute (NCI) | February 2016 | Phase 1 |
| NCT02431559 | Recruiting | Ovarian Cancer | Ludwig Institute for Cancer Research|MedImmune LLC|VentiR …more | November 2015 | Phase 1|Phase 2 |
| NCT02124850 | Recruiting | Squamous Cell Carcinoma of the Head and Neck | VentiRx Pharmaceuticals Inc. | September 2014 | Phase 1 |
| NCT01836029 | Active, not recruiting | Carcinoma, Squamous Cell of Head and Neck | VentiRx Pharmaceuticals Inc. | July 2013 | Phase 2 |
| NCT01666444 | Active, not recruiting | Epithelial Ovarian Cancer|Fallopian Tube Cancer|Primary Peritoneal Cancer | VentiRx Pharmaceuticals Inc.|Gynecologic Oncology Group | October 2012 | Phase 2 |
view more
Chemical Information
Download Motolimod (VTX-2337) SDF
| Molecular Weight (MW) | 458.6 |
|---|---|
| Formula | C28H34N4O2 |
| CAS No. | 926927-61-9 |
| Storage | 3 years -20℃powder |
|---|---|
| 6 months-80℃in solvent | |
| Synonyms | N/A |
| Solubility (25°C) * | In vitro | DMSO | 55 mg/mL warming (119.93 mM) |
|---|---|---|---|
| Ethanol | 15 mg/mL (32.7 mM) | ||
| Water | <1 mg/mL (<1 mM) | ||
| In vivo | |||
| * <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
|||
PATENT
formula (I).

((IE, 4E)-2-amino-N,N-dipropyl-8-(4-(pyrrolidine-l-carbonyl)phenyl)-3H-benzo[b]azepine-4-carboxamide (“Compound A”)). The crystalline form can be an unsolvated or solvated crystalline form of the compound of formula (I).
Also provided herein are compositions including the crystalline forms of the compound of formula (I) described herein, methods of making the crystalline forms, and methods of using the crystalline forms for the treatment of diseases, including, for example, cancer.
Further provided herein are methods of agonizing a Toll-like receptor using the crystalline forms of the compound of formula (I) described herein. In one aspect the method includes agonizing a Toll-like receptor (TLR8) by contacting TLR8 with an effective amount of a crystalline form of the compound formula (I) described herein, wherein the effective amount agonizes the TLR8.
PATENT
WO2007024612
https://www.google.com/patents/WO2007024612A2?cl=en
Example 10
Synthesis of ClE, 4E)-2-ammo-N,N-dipropyl-8-(4-rpyrrolidine-l-carbonyl)phenyl)-3H- benzorbiazepine-4-carboxamide C27)
Compound (27) was prepared from compound (24) by a method similar to that described in Example 2 to provide 49 mg (43%) of the desired compound. 1H NMR (CDCl3) δ 0.93 (t, 6H), 1.63-1.71 (m, 4H), 1.89 (m, 2H), 1.98 (m, 2H), 2.83 (s, 2H), 3.40-3.51 (m, 6H), 3.67 (t, 2H), 6.83 (s, IH), 7.3 (dd, IH), 7.35 (d, IH), 7.49 (d, IH)5 7.64 (q, 4H).
EXAMPLE 2 CLIP, QUANTITIES MAY VARY USE YOUR DISCRETION
Trimethylaluminum (0.34 mL of a 2.0 M solution in toluene) was added to bis(2- methoxyethyl)amine (92 mg, 0.69 mmol) in DCE (3 mL). After 10 minutes solid COMPD 24, 0.23 mmol) was added and the vessel was sealed and heated to 75 0C for 16-20 hours. Upon cooling the reaction was quenched with saturated Rochelle’s salt (2 mL) and after 20 minutes the mixture was partitioned between CH2Cl2 (50 mL) and brine (50 mL). The phases were separated and the aqueous was extracted with CH2Cl2 (2 x 20 mL). The combined organics were dried and concentrated. The crude material was purified via preparative TLC (2, 0.5 mm plates, eluting with 5-10% MeOH/CH2Cl2 with 4-6 drops of NH4OH)
Synthesis of (IE, 4E)-ethyl 2-ammo-8-(pyrrolidine-l-carbonyl)-3H-benzorb]azepine-4- carboxylate (24)
The reaction scheme for the synthesis of compound (24) is shown in Figure 4. Step A: Preparation of (E)-2-(4-bromo-2-nitrophenyl)-N,N-dimethylethenamine (18):
To a solution of l-methyl-2-nitro-4-bromobenzene (17) (29.86 g, 138.2 mmol) in toluene (200 niL) was added dimethylformamide dimethylacetal (17.52 g, 138.2 mmol). The mixture was heated to reflux for 14 hours. After cooling to room temperature the mixture was concentrated under vacuum and the resulting oil was immediately used in the next reaction. Step B: Preparation of 4-bromo-2-nitrobenzaldehyde (19): To a solution of crude (E)-
2-(4-bromo-2-nitrophenyl)-N,N-dimethylethenamine (35.5 g, 131 mmol) in THF (300 mL) and pH 7.2 phosphate buffer (300 mL) was added NaIO4 (56.0 g, 262 mmol). The solids were removed and the filter cake was washed with EtOAc (200 mL). The filtrate was washed with brine (2 X 100 mL), dried and concentrated. The concentrate was purified via flash chromatography (5% EtOAc/hexanes to 10% EtOAc/hexanes) to provide 4-bromo-2- nitrobenzaldehyde (8.41 g, 28% yield).
Step C: Preparation of (E)-ethyl 3-(4-bromo-2-nitrophenyl)-2-(cyanomethyl)acrylate (20): To a solution of 4-bromo-2-nitrobenzaldehyde (3.45 g, 15.0 mmol) in toluene (15 mL) was added α-cyanomethylcarboethoxyethylidene triphenylphosphorane (6.1O g, 15.7 mmol). The mixture was heated to 75 °C for 16 hours. The reaction was allowed to cool and the solvent was removed under vacuum. The concentrate was purified via flash chromatography (100% hexanes to 20% EtOAc) to yield (E)-ethyl 3-(4-bromo-2-nitrophenyl)-2- (cyanomethyl)acrylate (2.25 g, 44% yield) as an off white solid.
Step D: Preparation of (IE, 4E)-ethyl 2-ammo-8-bromo-3H-benzo|“b1azepine-4- carboxylate (21): To a solution of (E)-ethyl 3-(4-bromo-2-nitrophenyl)-2- (cyanomethyl)acrylate (1.00 g, 2.9 mmol) in acetic acid (25 mL) was added iron powder (1.10 g, 19.0 mmol). The mixture was heated to 90 °C for 5 hours. Upon cooling the acetic acid was removed under vacuum and the resulting semisolid was dissolved in 50% K2CO3 (100 mL) and EtOAc (100 mL). The mixture was filtered to remove insoluble material and the phases were separated. The aqueous phase was extracted with EtOAc (2 x 100 mL). The combined organics were dried and concentrated. The concentrate was purified via flash chromatography (Biotage 40m, 5% MeOH/CH2Cl2) to yield (lE,4E)-ethyl 2-amino-8-bromo- 3H-benzo[b] azepine-4-carboxylate (0.52 g, 57%).
Step E: Preparation of (IE. 4E)-ethyl-8-bromo-2-(tert-butoxycarbonyl)-3H- benzo FbI azepine-4-carboxylate (22) : To a CH2Cl2 (5 mL) solution containing (IE, 4E)-ethyl 2-amino-8-bromo-3H-benzo[b]azepine-4-carboxylate (198 mg, 0.640 mmol) was added Boc anhydride (140 mg, 0.640 mmol). The solution was stirred at room temperature for 72 hours. The reaction was concentrated to dryness and purified by column chromatography (Biotage 12m, 4:1 hexanes :EtO Ac) to provide (IE, 4E)-ethyl-8-bromo-2-(tert-butoxycarbonyl)-3H- benzo[b] azepine-4-carboxylate (245 mg, 94% yield) as a white solid. Step F: Preparation of (IE, 4E)-ethyl-2-(tert-butoxycarbonyl)-8-(pyrrolidine-l- carbonyl)-3H-benzo Fb] azepme-4-carboxylate (23) : To an ethanol solution (15 mL) containing K3PO4 (938 mg, 4.42 mmol), 4-(pyrrolidine-l-carbonyl)phenylboronic acid (785 mg, 3.58 mmol), and (IE, 4E)-ethyl-8-bromo-2-(tert-butoxycarbonyl)-3H-benzo[b]azepine-4- carboxylate (489 mg, 1.19 mmol), was added palladium acetate (80.5 mg, 0.358 mmol). The reaction was heated to 60 °C for 2 hours, then cooled to room temperature and concentrated to dryness. The brown oil was purified by preparative LC plate (100% EtOAc) to provide (lE,4E)-ethyl-2-(tert-butoxycarbonyl)-8-(pyrrolidine-l-carbonyl)-3H-benzo[b]azepine-4- carboxylate (277 mg, 46% yield) as a tan oil.
Step G: Preparation of (IE, 4E)-ethyl 2-amino-8-(pyrrolidine-l-carbonyl)-3H- benzoFbl azepine-4-carboxylate (24V (IE, 4E)-ethyl-2-(tert-butoxycarbonyl)-8-(pyrrolidine-l- carbonyl)-3H-benzo[b]azepine-4-carboxylate (110 mg, 0.218 mmol) was diluted with a 1:4 TFA:CH2C12 solution (4 mL). The reaction was stirred at room temperature for 1 hour, and then diluted with CH2Cl2. The organic phase was washed with 10% K2CO3 and brine (30 mL). The CH2Cl2 solution was dried over Na2SO4, filtered, and concentrated to provide (IE, 4E)-ethyl 2-amino-8-(pyrrolidine-l-carbonyl)-3H-benzo[b]azepine-4-carboxylate (88 mg, 81% yield) as a yellow solid. 1H NMR (CDCl3) δ 1.39 (t, 3H), 1.88-1.99 (m, 4H), 2.98 (s, 2H), 3.49-3.52 (m, 2H), 3.66-3.69 (m, 2H), 4.30-4.35 (m, 2H), 7.32 (d, IH), 7.46-7.49 (m, 2H), 7.60 (d, 2H) 7.67 (d, 2H), 7.84 (s, IH).
PATENT
WO2012045090
(assigned to VentiRx), claiming an aqueous composition comprising a TLR-8 agonist (ie motolimod) and an anti-cancer agent (eg doxorubicin, gemcitabine or cyclophosphamide), useful for treating cancer.
| Patent ID | Date | Patent Title |
|---|---|---|
| US2016045502 | 2016-02-18 | THERAPEUTIC BENEFIT OF SUBOPTIMALLY ADMINISTERED CHEMICAL COMPOUNDS |
| US2015182490 | 2015-07-02 | METHODS FOR TREATING TYROSINE-KINASE-INHIBITOR-RESISTANT MALIGNANCIES IN PATIENTS WITH GENETIC POLYMORPHISMS OR AHI1 DYSREGULATIONS OR MUTATIONS EMPLOYING DIANHYDROGALACTITOL, DIACETYLDIANHYDROGALACTITOL, DIBROMODULCITOL, OR ANALOGS OR DERIVATIVES THEREOF |
| US2014066432 | 2014-03-06 | Substituted Benzoazepines As Toll-Like Receptor Modulators |
| US2013236449 | 2013-09-12 | METHODS OF ENHANCING ANTIBODY-DEPENDENT CELLULAR CYTOTOXICITY |
| US2013018042 | 2013-01-17 | Toll-Like Receptor Agonist Formulations and Their Use |
| US8304407 | 2012-11-06 | 8-substituted benzoazepines as toll-like receptor modulators |
| US2012219615 | 2012-08-30 | Therapeutic Use of a TLR Agonist and Combination Therapy |
| US8242106 | 2012-08-14 | TOLL-LIKE RECEPTOR AGONIST FORMULATIONS AND THEIR USE |
| US8153622 | 2012-04-10 | 8-Substituted Benzoazepines as Toll-Like Receptor Modulators |
| US2012082658 | 2012-04-05 | Methods for the Treatment of Allergic Diseases |
//////Motolimod, VTX-2337, 莫托莫德 , мотолимод , موتوليمود , VTX 2337, VTX-378, 926927-61-9, phase 2, TLR-8 agonist
CCCN(CCC)C(=O)C1=CC2=C(C=C(C=C2)C3=CC=C(C=C3)C(=O)N4CCCC4)N=C(C1)N















Sorry, the comment form is closed at this time.