OR
ONL 1204
CAS 1349038-53-4
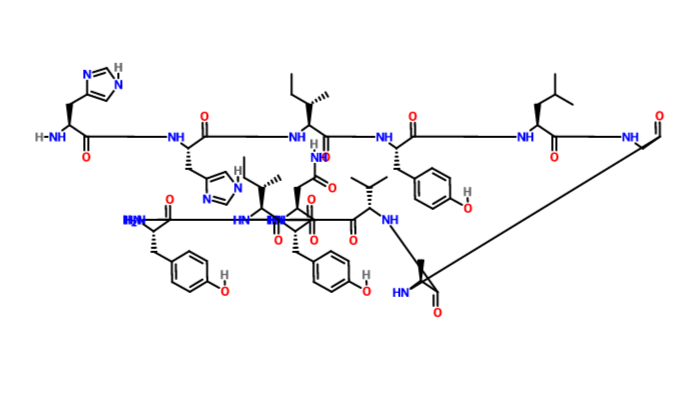
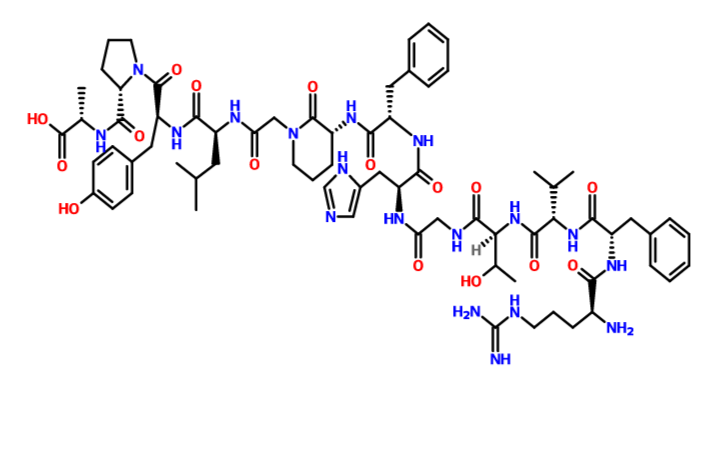
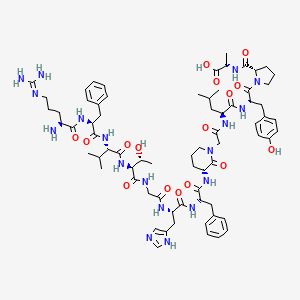
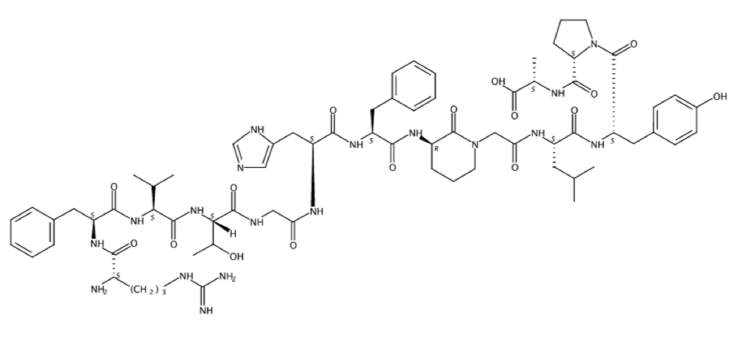
(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[2-[(3R)-3-[[(2S)-2-[[(2S)-2-[[2-[[(2S,3R)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]-3-phenylpropanoyl]amino]-3-methylbutanoyl]amino]-3-hydroxybutanoyl]amino]acetyl]amino]-3-(1H-imidazol-5-yl)propanoyl]amino]-3-phenylpropanoyl]amino]-2-oxopiperidin-1-yl]acetyl]amino]-4-methylpentanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]pyrrolidine-2-carbonyl]amino]propanoic acid
His-His- Ile-Tyr-Leu-Gly-Ala-Val-Asn-Tyr-Ile-Tyr-NH2
ONL Therapeutics Inc.
Fas receptor (CD95)
Peptide, Retinal detachment, OPTHALMIC DRUGS
C71 H100 N18 O16, 1461.66
L-Histidyl-L-histidyl-L-isoleucyl-L-tyrosyl-L-leucylglycyl-L-alanyl-L-valyl-L-asparaginyl-L-tyrosyl-L-isoleucyl-L-tyrosinamide
RFVTGHFXGL YPA
ORPHAN DRUG DESIGNATION DATA
His-His- Ile-Tyr-Leu-Gly-Ala-Val-Asn-Tyr-Ile-Tyr-NH2
01/13/2016
Treatment of retinal detachment
ONL Therapeutics, Inc
1600 Huron Parkway
Second Floor
Ann Arbor, Michigan 48109…….http://www.accessdata.fda.gov/scripts/opdlisting/oopd/OOPD_Results_2.cfm?Index_Number=501215
![]()
ONL1204, ONL’s lead therapeutic candidate, is a first-in-class small molecule peptide designed to protect key retinal cells, including photoreceptors, against the apoptosis (programmed cell death) that occurs in a range of retinal diseases and conditions. It is this death of these retinal cells that is the root cause of vision loss and the leading cause of blindness.
Researchers have shown that ONL1204 effectively inhibits the Fas pathway; one of the body’s primary mechanisms for inducing programmed cell death (apoptosis). Specifically, the compound’s activity inhibits the Fas receptor, blocks the activation of the Fas pathway, and prevents the apoptosis cascade which results in the death of key retinal cells, including photoreceptor.
While initial development efforts for ONL1204 are focused on retinal detachment, preclinicalin vivo data, along with a growing body of literature, support potential application in age-related macular degeneration (AMD) and other chronic retinal diseases. Combined, the estimated market for the initial indications that ONL plans to target is >$12 billion globally.
ONL Therapeutics, Inc., a biopharmaceutical company developing novel therapies for preserving sight in a range of retinal diseases, today announced that the United States Food and Drug Administration (FDA) has granted orphan drug designation to ONL1204 for the treatment of retinal detachment. ONL1204 is a novel, first-in-class small molecule peptide designed to protect key retinal cells, including photoreceptors, from cell death that occurs in a range of retinal diseases and conditions. Death of these retinal cells is the root cause of vision loss and the leading cause of blindness. ONL expects to advance ONL1204 into clinical trials for retinal detachment patients in 2016.
Retinal detachment occurs when the retina is separated from the underlying layer of cells called the retinal pigment epithelium (RPE). The RPE provides nutritional support to the highly-active photoreceptors in the retina. When there is a detachment, the photoreceptors no longer receive these nutrients and undergo cell death processes that dramatically impact a patient’s vision. Retinal detachments occur in approximately 50,000 people each year in the United States and affect people of all ages, although risk increases as people reach fifty years of age.
Patients experiencing a retinal detachment are normally treated by surgical reattachment of the retina to reconnect the photoreceptors with the RPE and prevent additional loss of vision. However, these procedures do not address the photoreceptor death and vision loss, which can be significant, that occurs prior to surgery. ONL1204 will be delivered to patients upon diagnosis and is intended to block photoreceptor cells from dying until surgery can be completed.
“When retinal detachments involve the center of vision called the macula, more than a third of patients have final best corrected vision of 20/60 or worse after successful surgery,” said David Zacks, M.D., Ph.D., co-founder and chief science officer of ONL Therapeutics. “Those are truly poor outcomes from successful surgeries. We are very pleased the FDA has recognized this need and that ONL is the only company to have received an orphan designation for this disease. It reinforces our belief that ONL1204 can play a key role in preventing vision loss in these patients by protecting their photoreceptors.”
The FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions that affect less than 200,000 individuals in the US. A drug candidate and its developer must meet several key criteria in order to qualify for, and obtain, orphan drug status. Once a drug has received orphan drug designation, the developer qualifies for a range of benefits, including federal grants, tax credits, reduction in certain regulatory fees, and the potential for seven years of market exclusivity for the drug following FDA marketing approval.
About ONL Therapeutics
ONL Therapeutics (ONL) is a biopharmaceutical company committed to protecting and improving the vision of patients with retinal disease. By advancing a novel breakthrough technology designed to protect key retinal cells from Fas-mediated cell death, ONL is pioneering an entirely new approach to preserving sight. The death of key retinal cells is the root cause of vision loss and leading cause of blindness, and is implicated in a wide range of retinal diseases, including retinal detachment and both the wet and dry forms of age related macular degeneration (AMD).
read
FDA grants orphan status for ONL Therapeutics’ ONL1204 to treat retinal detachment
The US Food and Drug Administration (FDA) has granted orphan drug designation for ONL Therapeutics’ first-in-class small molecule peptide, ONL1204, for the treatment of retinal detachment.
see,………https://newdrugapprovals.org/2016/02/17/onl-1204-a-small-molecule-peptide/
/////
N[C@@H](CCCNC(=N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)O)C(=O)NCC(=O)N[C@@H](Cc2cncn2)C(=O)N[C@@H](Cc3ccccc3)C(=O)N[C@@H]6CCCN(CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc4ccc(O)cc4)C(=O)N5CCC[C@H]5C(=O)N[C@@H](C)C(=O)O)C6=O
OR
CC(C)CC(C(=O)NC(CC1=CC=C(C=C1)O)C(=O)N2CCCC2C(=O)NC(C)C(=O)O)NC(=O)CN3CCCC(C3=O)NC(=O)C(CC4=CC=CC=C4)NC(=O)C(CC5=CN=CN5)NC(=O)CNC(=O)C(C(C)O)NC(=O)C(C(C)C)NC(=O)C(CC6=CC=CC=C6)NC(=O)C(CCCN=C(N)N)N
OR
C[C@@H](CC)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(=O)N)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](Cc3cncn3)N)Cc4cncn4)[C@@H](C)CC)C(C)C)C(=O)N[C@@H](Cc5ccc(O)cc5)C(N)=O













Sorry, the comment form is closed at this time.