
Dithiocarbamates: Reagents for the Removal of Transition Metals from Organic Reaction Media
http://pubs.acs.org/doi/abs/10.1021/op500336h

http://pubs.acs.org/doi/abs/10.1021/op500336h

1H NMR RABEPRAZOLE
RAB standard: (DMSOd6 400 MHz) δ 8.28 (d, 1H, J=5.56 Hz), 7.46 (m, 2H), 6.93 (d, 1H, J=5.68 Hz), 6.88 (m, 2H), 4.57 (AB, 2H, J=12.88 Hz), 4.10 (t, 2H, J=6.19 Hz), 3.49 (t, 2H, J=6.32 Hz), 3.25 (S, 3H), 2.17 (S, 3H), 1.98 (quin., 2H, J=6.19 Hz) 13C NMR (DMSOd6, 101 MHz) δ 162.61, 152.41, 147.95, 146.62, 121.74, 118.20, 117.32, 105.92, 68.30, 64.92, 59.64, 57.96, 28.67, 10.83.
The 1H spectrum for the RAB starting material is shown with peaks labelled and integrated. Data were collected on a 400 MHz NMR in DMSOd6, corrected to TMS by residual non-deuterated solvent.
Figure S13 COSY NMR spectrum of RAB
The COSY spectrum for the RAB starting material is shown. This was used to assign the methoxypropoxy carbon chain δ 1.98, 3.49 and 4.10. Data were collected on a 400 MHz NMR in DMSOd6, corrected to TMS by residual non-deuterated solvent.
Figure S14 HMBC spectrum of RAB
The spectrum shows the long range coupling of 1H to 13C nuclei. Coupling is observed between the 3-methyl group and the AB system, and between the various hydrogens on the alkyl chain.

Evaluation of antispasmodic activity of different Shodhit guggul using different shodhan process
Rachana Kamble, Sadhana Sathaye, DP Shah
University Institute of Chemical Technology, N. Parekh Marg, Matunga, Mumbai-400 019, India
According to ayurvedic texts shodhan vidhi is an important process which enhances the biological activity of a compound and reduces the toxicity at the same time. Before incorporating into formulations, guggul is processed using Shodhan vidhi involving different shodhan dravyas like gulvel, gomutra, triphala, dashmul. We have evaluated the antispasmodic activity of guggul on ileum of guinea pig and Wistar rats. The animals were sacrificed and ileum tissue of guinea pig and rat was isolated and tested for antispasmodic activity using different spasmogens like acetylcholine, histamine and barium chloride. It was observed that the different shodhit guggul (shudha guggul) i.e. processed using different shodhan vidhi, showed good antispasmodic activity as compared to Ashudha guggul. When acetylcholine was used as spasmogen, gulvel and triphala shodhit guggul showed good antispasmodic activity than other shodhit guggul. Thus shodhan vidhi enhances the therapeutic properties of guggul.
Kamble R, Sathaye S, Shah D P. Evaluation of antispasmodic activity of different Shodhit guggul using different shodhan process. Indian J Pharm Sci 2008;70:368-72
URL:
Kamble R, Sathaye S, Shah D P. Evaluation of antispasmodic activity of different Shodhit guggul using different shodhan process. Indian J Pharm Sci [serial online] 2008 [cited 2014 Dec 9];70:368-72. Available from: http://www.ijpsonline.com/text.asp?2008/70/3/368/43005


Associate professor of Pharmacy(Pharmacology) at Institute Of Chemical Technology

The new helical molecule (right) was due to the different length strands (blue and gray) a spatial arrangement of a (schematized in the middle), similar to the railing of a spiral staircase (left).
BASEL, SWITZERLAND: Chemists at the University of Basel have managed to twist a molecule in a novel way by different combined long molecular strands together. In this case, the longer strand winds like a stair railing around a central axis, and there is a mirror-image structure, which has special physical properties.
http://www.worldofchemicals.com/media/academy/new-novel-method-to-twist-molecule/7778.html
The 1H-NMR spectra that we have seen so far (of methyl acetate and para-xylene) are somewhat unusual in the sense that in both of these molecules, each set of protons generates a single NMR signal. In fact, the 1H-NMR spectra of most organic molecules contain proton signals that are ‘split’ into two or more sub-peaks. Rather than being a complication, however, this splitting behavior actually provides us with more information about our sample molecule.
Consider the spectrum for 1,1,2-trichloroethane. In this and in many spectra to follow, we show enlargements of individual signals so that the signal splitting patterns are recognizable.

The signal at 3.96 ppm, corresponding to the two Ha protons, is split into two subpeaks of equal height (and area) – this is referred to as adoublet. The Hb signal at 5.76 ppm, on the other hand, is split into three sub-peaks, with the middle peak higher than the two outside peaks – if we were to integrate each subpeak, we would see that the area under the middle peak is twice that of each of the outside peaks. This is called a triplet.
The source of signal splitting is a phenomenon called spin-spin coupling, a term that describes the magnetic interactions between neighboring, non-equivalent NMR-active nuclei. In our 1,1,2 trichloromethane example, the Ha and Hb protons are spin-coupled to each other. Here’s how it works, looking first at the Ha signal: in addition to being shielded by nearby valence electrons, each of the Ha protons is also influenced by the small magnetic field generated by Hb next door (remember, each spinning proton is like a tiny magnet). The magnetic moment of Hb will be aligned with B0 in (slightly more than) half of the molecules in the sample, while in the remaining half of the molecules it will be opposed to B0. The Beff ‘felt’ by Ha is a slightly weaker if Hb is aligned against B0, or slightly stronger if Hb is aligned with B0. In other words, in half of the molecules Ha is shielded by Hb (thus the NMR signal is shifted slightly upfield) and in the other half Ha isdeshielded by Hb(and the NMR signal shifted slightly downfield). What would otherwise be a single Ha peak has been split into two sub-peaks (a doublet), one upfield and one downfield of the original signal. These ideas an be illustrated by a splitting diagram, as shown below.

Now, let’s think about the Hbsignal. The magnetic environment experienced by Hb is influenced by the fields of both neighboring Haprotons, which we will call Ha1 and Ha2. There are four possibilities here, each of which is equally probable. First, the magnetic fields of both Ha1 and Ha2 could be aligned with B0, which would deshield Hb, shifting its NMR signal slightly downfield. Second, both the Ha1 and Ha2 magnetic fields could be aligned opposed to B0, which would shield Hb, shifting its resonance signal slightly upfield. Third and fourth, Ha1 could be with B0 and Ha2 opposed, or Ha1opposed to B0 and Ha2 with B0. In each of the last two cases, the shielding effect of one Haproton would cancel the deshielding effect of the other, and the chemical shift of Hb would be unchanged.

So in the end, the signal for Hb is a triplet, with the middle peak twice as large as the two outer peaks because there are two ways that Ha1and Ha2 can cancel each other out.
Now, consider the spectrum for ethyl acetate:

We see an unsplit ‘singlet’ peak at 1.833 ppm that corresponds to the acetyl (Ha) hydrogens – this is similar to the signal for the acetate hydrogens in methyl acetate that we considered earlier. This signal is unsplit because there are no adjacent hydrogens on the molecule. The signal at 1.055 ppm for the Hc hydrogens is split into a triplet by the two Hb hydrogens next door. The explanation here is the same as the explanation for the triplet peak we saw previously for 1,1,2-trichloroethane.
The Hbhydrogens give rise to a quartet signal at 3.915 ppm – notice that the two middle peaks are taller then the two outside peaks. This splitting pattern results from the spin-coupling effect of the three Hc hydrogens next door, and can be explained by an analysis similar to that which we used to explain the doublet and triplet patterns.
|
||
|---|---|---|
By now, you probably have recognized the pattern which is usually referred to as the n + 1 rule: if a set of hydrogens has n neighboring, non-equivalent hydrogens, it will be split into n + 1 subpeaks. Thus the two Hb hydrogens in ethyl acetate split the Hc signal into a triplet, and the three Hc hydrogens split the Hb signal into a quartet. This is very useful information if we are trying to determine the structure of an unknown molecule: if we see a triplet signal, we know that the corresponding hydrogen or set of hydrogens has two `neighbors`. When we begin to determine structures of unknown compounds using 1H-NMR spectral data, it will become more apparent how this kind of information can be used.
Three important points need to be emphasized here. First, signal splitting only occurs between non-equivalent hydrogens – in other words, Ha1 in 1,1,2-trichloroethane is not split by Ha2, and vice-versa.

Second, splitting occurs primarily between hydrogens that are separated by three bonds. This is why the Ha hydrogens in ethyl acetate form a singlet– the nearest hydrogen neighbors are five bonds away, too far for coupling to occur.

Occasionally we will see four-bond and even 5-bond splitting, but in these cases the magnetic influence of one set of hydrogens on the other set is much more subtle than what we typically see in three-bond splitting (more details about how we quantify coupling interactions is provided in section 5.5B). Finally, splitting is most noticeable with hydrogens bonded to carbon. Hydrogens that are bonded to heteroatoms (alcohol or amino hydrogens, for example) are coupled weakly – or not at all – to their neighbors. This has to do with the fact that these protons exchange rapidly with solvent or other sample molecules.
Below are a few more examples of chemical shift and splitting pattern information for some relatively simple organic molecules.



|
||
|---|---|---|
Chemists quantify the spin-spin coupling effect using something called the coupling constant, which is abbreviated with the capital letterJ. The coupling constant is simply the difference, expressed in Hz, between two adjacent sub-peaks in a split signal. For our doublet in the 1,1,2-trichloroethane spectrum, for example, the two subpeaks are separated by 6.1 Hz, and thus we write 3Ja-b = 6.1 Hz.

The superscript 3 tells us that this is a three-bond coupling interaction, and the a-b subscript tells us that we are talking about coupling between Ha and Hb. Unlike the chemical shift value, the coupling constant, expressed in Hz, is the same regardless of the applied field strength of the NMR magnet. This is because the strength of the magnetic moment of a neighboring proton, which is the source of the spin-spin coupling phenomenon, does not depend on the applied field strength.
When we look closely at the triplet signal in 1,1,2-trichloroethane, we see that the coupling constant – the `gap` between subpeaks – is 6.1 Hz, the same as for the doublet. This is an important concept! The coupling constant 3Ja-b quantifies the magnetic interaction between the Ha and Hb hydrogen sets, and this interaction is of the same magnitude in either direction. In other words, Ha influences Hb to the same extent that Hb influences Ha. When looking at more complex NMR spectra, this idea of reciprocal coupling constants can be very helpful in identifying the coupling relationships between proton sets.
Coupling constants between proton sets on neighboring sp3-hybridized carbons is typically in the region of 6-8 Hz. With protons bound to sp2-hybridized carbons, coupling constants can range from 0 Hz (no coupling at all) to 18 Hz, depending on the bonding arrangement.

For vinylic hydrogens in a trans configuration, we see coupling constants in the range of 3J = 11-18 Hz, while cis hydrogens couple in the 3J= 6-15 Hz range. The 2-bond coupling between hydrogens bound to the same alkene carbon (referred to as geminal hydrogens) is very fine, generally 5 Hz or lower. Ortho hydrogens on a benzene ring couple at 6-10 Hz, while 4-bond coupling of up to 4 Hz is sometimes seen between meta hydrogens.

Fine (2-3 Hz) coupling is often seen between an aldehyde proton and a three-bond neighbor. Table 4 lists typical constant values.
In all of the examples of spin-spin coupling that we have seen so far, the observed splitting has resulted from the coupling of one set of hydrogens to just one neighboring set of hydrogens. When a set of hydrogens is coupled to two or more sets of nonequivalent neighbors, the result is a phenomenon called complex coupling. A good illustration is provided by the 1H-NMR spectrum of methyl acrylate:

First, let’s first consider the Hc signal, which is centered at 6.21 ppm. Here is a closer look:

With this enlargement, it becomes evident that the Hc signal is actually composed of four sub-peaks. Why is this? Hc is coupled to both Haand Hb , but with two different coupling constants. Once again, a splitting diagram can help us to understand what we are seeing. Ha istrans to Hc across the double bond, and splits the Hc signal into a doublet with a coupling constant of 3Jac = 17.4 Hz. In addition, each of these Hc doublet sub-peaks is split again by Hb (geminal coupling) into two more doublets, each with a much smaller coupling constant of2Jbc = 1.5 Hz.

The result of this `double splitting` is a pattern referred to as a doublet of doublets, abbreviated `dd`.
The signal for Ha at 5.95 ppm is also a doublet of doublets, with coupling constants 3Jac= 17.4 Hz and 3Jab = 10.5 Hz.

The signal for Hb at 5.64 ppm is split into a doublet by Ha, a cis coupling with 3Jab = 10.4 Hz. Each of the resulting sub-peaks is split again by Hc, with the same geminal coupling constant 2Jbc = 1.5 Hz that we saw previously when we looked at the Hc signal. The overall result is again a doublet of doublets, this time with the two `sub-doublets` spaced slightly closer due to the smaller coupling constant for the cisinteraction. Here is a blow-up of the actual Hbsignal:

|
||
|---|---|---|
When constructing a splitting diagram to analyze complex coupling patterns, it is usually easier to show the larger splitting first, followed by the finer splitting (although the reverse would give the same end result).
When a proton is coupled to two different neighboring proton sets with identical or very close coupling constants, the splitting pattern that emerges often appears to follow the simple `n + 1 rule` of non-complex splitting. In the spectrum of 1,1,3-trichloropropane, for example, we would expect the signal for Hb to be split into a triplet by Ha, and again into doublets by Hc, resulting in a ‘triplet of doublets’.

Ha and Hc are not equivalent (their chemical shifts are different), but it turns out that 3Jab is very close to 3Jbc. If we perform a splitting diagram analysis for Hb, we see that, due to the overlap of sub-peaks, the signal appears to be a quartet, and for all intents and purposes follows the n + 1 rule.

For similar reasons, the Hc peak in the spectrum of 2-pentanone appears as a sextet, split by the five combined Hb and Hd protons. Technically, this ‘sextet’ could be considered to be a ‘triplet of quartets’ with overlapping sub-peaks.

| http://newdrugapprovals.org/ |
|---|
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO |
In many cases, it is difficult to fully analyze a complex splitting pattern. In the spectrum of toluene, for example, if we consider only 3-bond coupling we would expect the signal for Hb to be a doublet, Hd a triplet, and Hc a triplet.

In practice, however, all three aromatic proton groups have very similar chemical shifts and their signals overlap substantially, making such detailed analysis difficult. In this case, we would refer to the aromatic part of the spectrum as a multiplet.
When we start trying to analyze complex splitting patterns in larger molecules, we gain an appreciation for why scientists are willing to pay large sums of money (hundreds of thousands of dollars) for higher-field NMR instruments. Quite simply, the stronger our magnet is, the more resolution we get in our spectrum. In a 100 MHz instrument (with a magnet of approximately 2.4 Tesla field strength), the 12 ppm frequency ‘window’ in which we can observe proton signals is 1200 Hz wide. In a 500 MHz (~12 Tesla) instrument, however, the window is 6000 Hz – five times wider. In this sense, NMR instruments are like digital cameras and HDTVs: better resolution means more information and clearer pictures



Pimobendan (INN is a veterinary medication manufactured by Boehringer Ingelheim under the trade names Vetmedin and Acardi) or pimobendane. It is a calcium sensitizer with positive inotropic and vasodilator effects. It is also a selective inhibitor ofphosphodiesterase III (PDE3).
Pimobendan is used in the management of heart failure in dogs, most commonly caused by myxomatous mitral valve disease (also known as endocardiosis), or dilated cardiomyopathy.[1] Research has shown that pimobendan increases survival time and improves quality of life in patients with congestive heart failure secondary to mitral valve disease when compared with benazepril, anangiotensin-converting-enzyme (ACE) inhibitor.[2] Under the trade name Acardi, it is available for human use in Japan.[3]
Pimobendan is a positive inotrope. It sensitizes and increases the binding efficiency of cardiac myofibril to the calcium ions that are already present without increasing the consumption of oxygen and energy. Pimobendan also causes peripheral vasodilation by inhibiting the function of phosphodiesterase III. This results in decreased pressure, translating into smaller cardiac preload andafterload (decreases the failing heart’s workload).
Pimobendan is absorbed rapidly when given via the oral route and has a bioavailability of 60-65%. It is metabolized into its active form by the liver. The half-life of pimobendan in the blood is 0.4 hours and the half-life of its metabolite is 2 hours. Elimination is by excretion in the bile and then feces. Pimobendan is 90–95% bound to plasma proteins in circulation. This has implications in patients suffering from low blood protein levels (hypoproteinemia/hypoalbuminemia) and with patients that are on concurrent therapies that are also highly protein bound.
Pimobendan is often used in combination with three other drugs to palliate dogs with heart disease and reduce clinical signs of disease. These are:
Other drugs may also be used as required to manage certain arrhythmias that are often associated with heart disease.
Pimobendan can be synthesized beginning with anisoyl chloride.
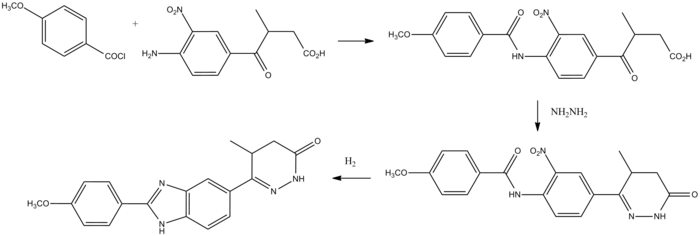
Pimobendan synthesis:[4]



 |
|
| Systematic (IUPAC) name | |
|---|---|
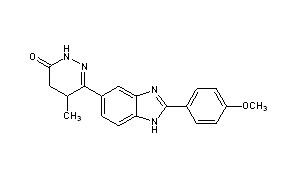
| (RS)-6-[2-(4-methoxyphenyl)-1H-benzimidazol-5-yl]-5-methyl-4,5-dihydropyridazin-3(2H)-one | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Legal status |
|
| Routes | Oral |
| Pharmacokinetic data | |
| Bioavailability | 60 to 65% |
| Half-life | 0.4 hours |
| Excretion | In feces |
| Identifiers | |
| CAS number | 74150-27-9 |
| ATCvet code | QC01CE90 |
| PubChem | CID 4823 |
| ChemSpider | 4657 |
| UNII | 34AP3BBP9T |
| KEGG | D01133 |
| ChEMBL | CHEMBL24646 |
| Chemical data | |
| Formula | C19H18N4O2 |
| Mol. mass | 334.37 g/mol |

Green Chem., 2015, Advance Article
DOI: 10.1039/C4GC02033B, Paper
Jiayin Hu, Jun Ma, Zhaofu Zhang, Qinggong Zhu, Huacong Zhou, Wenjing Lu, Buxing Han
CO2 can react with various propargylic amines to form 3,4,5-trisubstituted oxazolones catalyzed by the active, selective and stable ionic liquids.
Production of value-added chemicals using carbon dioxide (CO2) as a feedstock is favorable to the sustainable development of the chemical industry. In this work, we have discovered for the first time that CO2 can react with propargylic amines to produce 3,4,5-trisubstituted oxazolones, a class of very useful chemicals. It was found that the ionic liquid (IL) 1-butyl-3-methylimidazolium acetate ([Bmim][OAc]) can catalyze the reactions efficiently at atmospheric pressure under metal-free conditions. It was also found that [Bmim][OAc] and IL 1-butyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide ([Bmim][Tf2N]) have an excellent synergistic effect for promoting the reactions. The [Bmim][OAc]/[Bmim][Tf2N] catalytic system can be reused at least five times without loss in catalytic activity and selectivity. The reaction mechanism was proposed on the basis of density functional theory (DFT) calculation and the experimental results.


 |
Molecular Formula: C20H24N2O2 Molecular Weight: 324.417
CAS Number: 56-54-2 |
Quinine (US /ˈkwaɪnaɪn/, UK /ˈkwɪniːn/ or /kwɪˈniːn/ kwin-een) is a natural white crystalline alkaloid having antipyretic (fever-reducing),antimalarial, analgesic (painkilling), and anti-inflammatory properties and a bitter taste. It is a stereoisomer of quinidine, which, unlike quinine, is an antiarrhythmic. Quinine contains two major fused-ring systems: the aromatic quinoline and the bicyclic quinuclidine.
Quinine occurs naturally in the bark of the cinchona tree, though it has also been synthesized in the laboratory. The medicinal properties of the cinchona tree were originally discovered by the Quechua, who are indigenous to Peru and Bolivia; later, the Jesuitswere the first to bring cinchona to Europe.
Quinine was the first effective Western treatment for malaria caused by Plasmodium falciparum, appearing in therapeutics in the 17th century. It is pre-dated as a malarial treatment by the Chinese herbalist’s use of Artemisia annua, described in a 4th-century text, a plant from which the antimalarial drug artemisinin was derived. It remained the antimalarial drug of choice until the 1940s, when other drugs such as chloroquine that have fewer unpleasant side effects replaced it. Since then, many effective antimalarials have been introduced, although quinine is still used to treat the disease in certain critical circumstances, such as severe malaria, and in impoverished regions due to its low cost. Quinine is available with a prescription in the United States and “over-the-counter” (in minute quantities) in tonic water. Quinine is also used to treat lupus and arthritis. Quinine was also frequently prescribed in the US as an off-label treatment for nocturnal leg cramps, but this has become less prevalent due to a Food and Drug Administration statement warning against the practice.[2]
Quinine is highly fluorescent (quantum yield ~0.58) in 0.1 M sulfuric acid solution and it is widely used as a standard for fluorescence quantum yield measurement.[3][4] It is on the World Health Organization’s List of Essential Medicines, a list of the most important medications needed in a basic health system.[5]



As of 2006, quinine is no longer recommended by the WHO (World Health Organization), as first-line treatment for malaria, and should be used only when artemisinins are not available.[6][7][8][9]
Quinine is a basic amine and is usually presented as a salt. Various existing preparations include the hydrochloride, dihydrochloride,sulfate, bisulfate and gluconate. This makes quinine dosing complicated, since each of the salts has a different weight.
The following amounts of each salt form contain equal amounts of quinine:
All quinine salts may be given orally or intravenously (IV); quinine gluconate may also be given intramuscularly (IM) or rectally (PR).[10][11] The main problem with the rectal route is the dose can be expelled before it is completely absorbed; in practice, this is corrected by giving a half dose again.
In the United States, quinine sulfate is commercially available in 324-mg tablets under the brand name Qualaquin; the adult dose is two tablets every eight hours. No injectable preparation of quinine is licensed in the US; quinidine is used instead.[12][13]
13C NMR

The correct structure is at position 7.

Best 10 structures in decreasing rating (structure ID shown in parentheses):
1: 0.957622 ( 102439) 2: 0.957340 ( 3499717) 3: 0.955644 ( 65753)
4: 0.955078 ( 847715) 5: 0.953062 ( 6336167) 6: 0.953047 ( 585971)
7: 0.952892 ( 1065) 8: 0.950814 ( 8547) 9: 0.949068 ( 934598)
10: 0.948731 ( 2037943)
.gif)


The correct structure is at position 1.

Best 10 structures in decreasing rating (structure ID shown in parentheses):
1: 0.937466 ( 1065) 2: 0.915501 ( 6336167) 3: 0.900599 ( 3499717)
4: 0.897815 ( 183259) 5: 0.895740 ( 3083557) 6: 0.894629 ( 101764)
7: 0.894536 ( 84495) 8: 0.891677 ( 4835740) 9: 0.890939 ( 3809868)
10: 0.889296 ( 5191849)

Automated VerifyIt Proton Assignments:
|
Automated VerifyIt Carbon Assignments:
|







 |
Most likely structure (out of 277 possible ones) by agreement with carbon chemical shift prediction |
Quinine is used as an antimalaria drug.
Before performing a full structure elucidation, consider running FindIt. Only the molecular formula, proton, and/or (potentially only protonated) carbon shift information are needed.
At natural abundance, a 1D carbon spectrum is 5,700 times less sensitive to acquire than a 1D proton spectrum. Acquiring the shown HSQC and HMBC spectra instead is still faster than acquiring one 1D carbon spectrum. The full quinine structure elucidation is demonstrated using about 200 micro grams of sample. Only the shown NMR data from a room-temperature 1 mm capillary probe are used. No molecular formula (MF) or information from other spectroscopic methods are needed.
Quinine[34] is an effective muscle relaxant, long used by the Quechua, who are indigenous to Peru, to halt shivering due to low temperatures. The Peruvians would mix the ground bark of cinchona trees with sweetened water to offset the bark’s bitter taste, thus producing tonic water.
Quinine has been used in unextracted form by Europeans since at least the early 17th century. It was first used to treat malaria in Rome in 1631. During the 17th century, malaria was endemic to the swamps and marshes surrounding the city of Rome. Malaria was responsible for the deaths of severalpopes, many cardinals and countless common Roman citizens. Most of the priests trained in Rome had seen malaria victims and were familiar with theshivering brought on by the febrile phase of the disease. The Jesuit brother Agostino Salumbrino (1561–1642), an apothecary by training who lived inLima, observed the Quechua using the bark of the cinchona tree for that purpose. While its effect in treating malaria (and hence malaria-induced shivering) was unrelated to its effect in controlling shivering from rigors, it was still a successful medicine for malaria. At the first opportunity, Salumbrino sent a small quantity to Rome to test as a malaria treatment. In the years that followed, cinchona bark, known as Jesuit’s bark or Peruvian bark, became one of the most valuable commodities shipped from Peru to Europe. When King Charles II was cured of malaria at the end of the 17th Century with quinine, it became popular in London.[35] It remained the antimalarial drug of choice until the 1940s, when other drugs took over.[36]
The form of quinine most effective in treating malaria was found by Charles Marie de La Condamine in 1737.[37][38] Quinine was isolated and named in 1820 by French researchers Pierre Joseph Pelletier and Joseph Bienaimé Caventou.[39] The name was derived from the original Quechua (Inca) word for the cinchona tree bark, quina or quina-quina, which means “bark of bark” or “holy bark”. Prior to 1820, the bark was first dried, ground to a fine powder, and then mixed into a liquid (commonly wine) which was then drunk. Large-scale use of quinine as a prophylaxis started around 1850.
Quinine also played a significant role in the colonization of Africa by Europeans. Quinine had been said to be the prime reason Africa ceased to be known as the “white man’s grave”. A historian has stated, “it was quinine’s efficacy that gave colonists fresh opportunities to swarm into the Gold Coast, Nigeria and other parts of west Africa”.[40]
To maintain their monopoly on cinchona bark, Peru and surrounding countries began outlawing the export of cinchona seeds and saplings beginning in the early 19th century. The Dutch government persisted in its attempt to smuggle the seeds, and by the 1930s Dutch plantations in Java were producing 22 million pounds of cinchona bark, or 97% of the world’s quinine production.[40] During World War II, Allied powers were cut off from their supply of quinine when the Germans conquered the Netherlands and the Japanese controlled the Philippines and Indonesia. The United States had managed to obtain four million cinchona seeds from the Philippines and began operating cinchona plantations inCosta Rica. Nonetheless, such supplies came too late; tens of thousands of US troops in Africa and the South Pacific died due to the lack of quinine.[40] Despite controlling the supply, the Japanese did not make effective use of quinine, and thousands of Japanese troops in the southwest Pacific died as a result.[41][42][43][44]
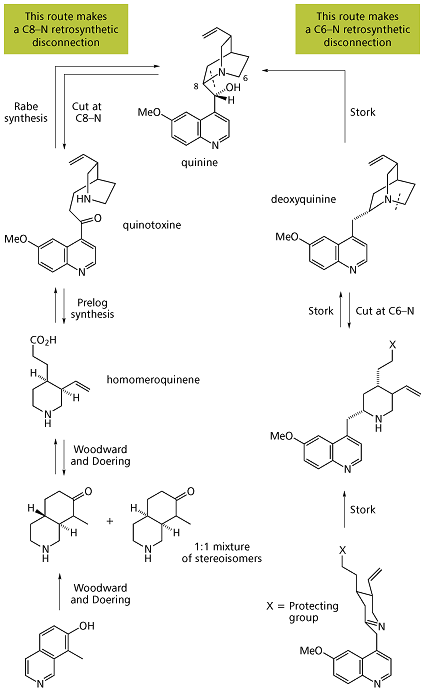
Cinchona trees remain the only economically practical source of quinine. However, under wartime pressure, research towards its synthetic production was undertaken. A formal chemical synthesis was accomplished in 1944 by American chemists R.B. Woodward and W.E. Doering.[45] Since then, several more efficient quinine total syntheses have been achieved,[46] but none of them can compete in economic terms with isolation of the alkaloid from natural sources. The first synthetic organic dye, mauveine, was discovered by William Henry Perkin in 1856 while he was attempting to synthesize quinine.
In total synthesis, the Quinine total synthesis describes the efforts in synthesis of quinine over a 150 year period. The development of synthetic quinine is considered a milestone in organic chemistry although it has never been produced industrially as a substitute for natural occurring quinine. The subject has also been attended with some controversy: in 2001 Gilbert Stork published the first stereoselective quinine synthesis and he shed doubt (calling it a myth) on the earlier claim in 1944 by Bob Woodward and William Doering on account that they had obtained not quinine but a precursor molecule. In 2001, an editorial in Chemical & Engineering Newssupported Storks claim but according to a critical 30 page review in this matter published in 2007 in Angewandte Chemie the Woodward/Doering claim is valid.

The aromatic component of the quinine molecule is a quinoline with a methoxy substituent. The amine component has a quinuclidine skeleton and the methylene bridge in between the two components has a hydroxyl group. The substituent at the carbon-3 position is a vinyl group. The molecule is optically active with five stereogenic centers (the N1 and C4 constituting a single asymmetric unit), making synthesis potentially difficult because it is one of 16 stereoisomers.




The Stork quinine synthesis starts from chiral (S)-4-vinylbutyrolactone 1. The compound is obtained by chiral resolution and in fact, in the subsequent steps all stereogenic centers are put in place by chiral induction: the sequence does not contain asymmetric steps.
 |
 |
|
| Stork quinine synthesis | Introducing C8 and nitrogen |
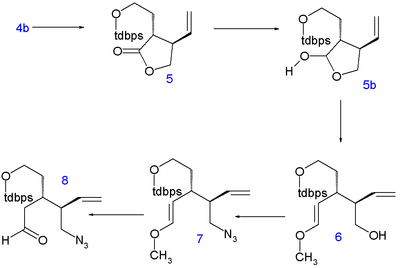
The lactone is ring-opened with diethylamine to amide 2 and its hydroxyl group is protected as a tert-butyldimethyl (TBS) silyl ether in 3. The C5 and C6 atoms are added as tert-butyldiphenylsilyl (TBDPS) protected iodoethanol in a nucleophilic substitution of acidic C4 with LDA at -78°C to 4 with correct stereochemistry. Removal of the silyl protecting group with p-toluenesulfonic acid to alcohol 4b and ring-closure by azeotropic distillation returns the compound to lactone 5 (direct alkylation of 1 met with undisclosed problems).
The lactone is then reduced to the lactol 5b with diisobutylaluminum hydride and its liberated aldehyde reacts in a Wittig reaction with methoxymethylenetriphenylphosphine(delivering the C8 atom) to form enol ether 6. The hydroxyl group is replaced in a Mitsunobu reaction by an azide group with diphenylphosphoryl azide in 7 and acid hydrolysis yields the azido aldehyde 8.
 |
 |
|
| First ring closure | Second ring closure |
The methyl group in 6-methoxy-4-methylquinoline 9 is sufficiently acidic for nucleophilic addition of its anion (by reaction with LDA) to the aldehyde group in 8 to form 10 as a mixture of epimers. This is of no consequence for stereocontrol because in the next step the alcohol is oxidized in a Swern oxidation to ketone 11. A Staudinger reaction withtriphenylphosphine closes the ring between the ketone and the azide to the tetrahydropyridine 12. The imine group in this compound is reduced to the amine 13 with sodium borohydride with the correct stereospecificity. The silyl protecting group is removed with hydrogen fluoride to alcohol 14 and then activated as an mesyl leaving group by reaction with mesyl chloride in pyridine which enables the third ring closure to 15. In the final step the C9 hydroxyl group was introduced by oxidation with sodium hydride, dmso and oxygen with quinine to epiquinine ratio of 14:1.
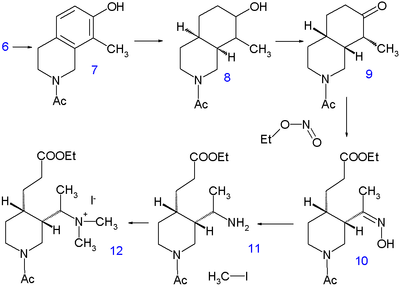
The 1944 Woodward / Doering synthesis starts from 7-hydroxyisoquinoline 3 for the quinuclidine skeleton which is somewhat counter intuitive because one goes from a stable heterocyclic aromat to a completely saturated bicyclic ring. This compound (already known since 1895) is prepared in two steps.
 |
 |
|
| Woodward/Doering quinine synthesis part I | Part II |
The first reaction step is condensation reaction of 3-hydroxybenzaldehyde 1 with (formally) the diacetal of aminoacetaldehyde to the imine 2 and the second reaction step is cyclization in concentrated sulfuric acid. Isoquinoline 3 is then alkylated in another condensation by formaldehyde and piperidine and the product is isolated as the sodium salt of4.
 |
|
| Woodward/Doering quinine synthesis part III |
Hydrogenation at 220°C for 10 hours in methanol with sodium methoxide liberates the piperidine group and leaving the methyl group in 5 with already all carbon and nitrogen atoms accounted for. A second hydrogenationtakes place with Adams catalyst in acetic acid to tetrahydroisoquinoline 6. Further hydrogenation does not take place until the amino group is acylated with acetic anhydride in methanol but by then 7 is again hydrogenated with Raney nickel in ethanol at 150°C under high pressure to decahydroisoquinoline 8. The mixture of cis and trans isomers is then oxidized by chromic acid in acetic acid to the ketone 9. Only the cis isomer crystallizes and used in the next reaction step, a ring opening with the alkyl nitrite ethyl nitrite with sodium ethoxide in ethanol to10 with a newly formed carboxylic ester group and an oxime group. The oxime group is hydrogenated to theamine 11 with platinum in acetic acid and alkylation with iodomethane gives the quaternary ammonium salt 12and subsequently the betaine 13 after reaction with silver oxide.
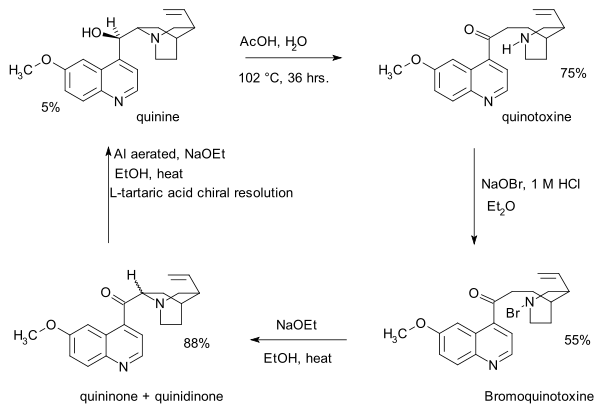
Quinine’s vinyl group is then constructed by Hofmann elimination with sodium hydroxide in water at 140°C. This process is accompanied by hydrolysis of both the ester and the amide group but it is not the free amine that is isolated but the urea 14 by reaction with potassium cyanate. In the next step the carboxylic acid group isesterified with ethanol and the urea group replaced with a benzoyl group. The final step is a claisen condensation of 15 with ethyl quininate 16, which after acidic workup yields racemic quinotoxine 17. The desired enantiomer is obtained by chiral resolution with the chiral dibenzoyl ester of Tartaric acid. The conversion of this compound to quinine is based on the Rabe/Kindler chemistry discussed in the timelime.
-alpha-fluoro-11-beta,17-alpha,21-trihydroxy-16-alpha-methylpregna-1,4-diene-3,20-dione; 9-alpha-fluoro-16-alpha-methylprednisolone; 9alpha-Fluoro-11beta,17alpha,21-trihydroxy-16alpha-methylpregn-1,4-diene-3,20-dione
CAS 50-02-2

1H NMR


IR


13C NMR


MASS

