Imigliptin
- CAS OF FREE BASE 1314944-07-4
- C21 H24 N6 O
- Benzonitrile, 2-[[7-[(3R)-3-amino-1-piperidinyl]-2,3-dihydro-3,5-dimethyl-2-oxo-1H-imidazo[4,5-b]pyridin-1-yl]methyl]-
Sihuan Pharmaceutical
Imigliptin dihydrochloride is an orally-available dipeptidyl peptidase IV (CD26; DPP-IV; DP-IV) inhibitor in phase I clinical trials at Sihuan Pharmaceutical for the treatment of type 2 diabetes.
………………………………………………………………
http://www.google.com/patents/EP2524917A1?cl=en
(R)-2-[[7-(3-aminopiperidin-1-yl)-3,5-dimethyl-2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl]methyl]benzonitrile AS TFA SALT
- 1314944-08-5 CAS
- C21 H24 N6 O . C2 H F3 O2
- Benzonitrile, 2-[[7-[(3R)-3-amino-1-piperidinyl]-2,3-dihydro-3,5-dimethyl-2-oxo-1H-imidazo[4,5-b]pyridin-1-yl]methyl]-, 2,2,2-trifluoroacetate (1:1)
………………………………………………………………………….
LEAD compd 1 as above ……….cas ………1314943-88-8
- C19 H19 N5 O2
- Benzonitrile, 2-[[7-[(3R)-3-amino-1-piperidinyl]-2-oxooxazolo[5,4-b]pyridin-1(2H)-yl]methyl]-
………………………………………
SEE POLYMORPHS
EP2730575A1, WO2013007167A1
CN 102863440
http://www.google.com/patents/CN102863440A?cl=en
Dipeptidyl peptidase-IV (DPP-IV) inhibitors are a new generation of oral treatment of type 2 diabetes by enhancing the role of incretin activity, a non-insulin therapy. With conventional medicine for treating diabetes compared, DPP-IV inhibitors have not weight gain and edema and other adverse reactions. [0003] The compound shown in formula ⑴ (R) -2 – [[7 – (3 – amino-piperidine-I-yl) -3,5 – dimethyl-2 – oxo-2 ,3 – dihydro- -IH-imidazo [4,5-b] pyridin-I-yl] methyl] benzonitrile (referred to as the specification of compound A, in the patent application CN201010291056. 9 already described) is a DPP-IV inhibitor compounds , the DPP-IV has a strong inhibitory effect and high selectivity.
V
[0004] formula ⑴
[0005] In the crystalline drug development research is very important, compound crystal form, will result in its stability, solubility and other properties are different. Therefore, the inventors of the compound or its salt polymorph A lot of research carried out, whereby it was confirmed, and the invention of the compound A crystalline salt.
3, Invention
[0006] The object of the present invention is to solve the above problems and to provide better stability, better maneuverability, good bioavailability and solubility of the compound A or a salt thereof and method for preparing the crystalline form.
[0007] The present invention provides formula (I), the compound A dihydrochloride salt polymorph I: using Cu-K α radiation, to angle 2 Θ (°) represents an X-ray powder diffraction at 8. 7 ± 0. 2 °, 19.4 ± 0.2 °, 23. 5 ± 0. 2 °, 27. 2 ± 0. 2 ° at a characteristic peaks.
Butterfly NC N
[0008] formula ⑴
[0009] A compound of the dihydrochloride salt polymorph I, with Cu-Ka radiation, to angle 2 Θ (°) represents an X-ray powder diffraction peaks in addition to the features described above, it also at 12. 5 ± 0. 2 °, 22. 5 ± 0. 2 °, 25. 5 ± 0.2 ° at a characteristic peaks.
[0010] A compound of the dihydrochloride salt polymorph I, with Cu-κα exposed to radiation angle 2 Θ (°) represents an X-ray powder diffraction peaks in addition to the features described above, it also at 11.7 ± 0.2 °, 14.6 ± 0.2 °,
26. O ± 0.2 ° at a characteristic peak.
[0011] The present invention also provides the compound A dihydrochloride Preparation of polymorph I.
[0012] Compound A was dissolved in an organic solvent, and temperature, was added dropwise a stoichiometric ratio of hydrochloric acid, after the addition was complete stirring, filtered and dried to give the dihydrochloride salt of Compound A crystalline form I.
……………………………………………….
http://www.google.com/patents/EP2524917A1?cl=en
0r
WO 2011085643
-
Diabetes mellitus is a systemic chronic metabolic disease caused by a blood glucose level higher than normal level due to loss of blood glucose control. It is basically classified into four categories, including: type I (insulin-dependent) and type II (non-insulin-dependent), the other type and gestational diabetes. Type I and type II diabetes are primary diabetes, which are the two most common forms caused by the interaction of genetic and environmental factors. The cause of diabetes is very complicated, but in the final analysis, is due to absolute or relative insulin deficiency, or insulin resistance. It is characterized by the metabolic disorder of carbohydrate, protein, fat, electrolytes and water caused by absolute or relative insulin deficiency and the reduced sensitivity of target cells to insulin.
-
In recent years, because of the improvement of living level, changes in the diet structure, the increasingly intense pace of life and lifestyle of less exercise and many other factors, the global incidence of diabetes is rapidly increasing, so that diabetes has become the third chronic disease which has a serious threat to human health next to tumor and cardiovascular diseases. Presently, the number of the patients suffering from diabetes has exceeded 120 million in the world, and the number in our country is the second largest in the world. According to statistics, up to 40 million people have been diagnosed as diabetes in China, and the number of the patients is increasing at a rate of 1 million per year. Among them, patients having type I and type II diabetes accounted for 10% and 90% respectively. Diabetes has become the increasingly concerned public health issue.
-
The main drugs currently used for the treatment of type I diabetes are insulin preparations and their substitutes; for the treatment of type II diabetes, the main drugs are oral hypoglycemic agents, generally divided into sulfonylureas, biguanides, traditional Chinese medicine preparations, other hypoglycemic agents, and auxiliary medication. Although these drugs have good effects, they can not maintain long-term efficacy in reducing the high blood glucose, and can not effectively alleviate the condition against the cause of diabetes. Many of the anti-diabetic drugs can well control the blood glucose at the beginning, but their efficacy can not be maintained when the treatment using such drugs are continuously used. It is one of the main reasons why combination therapies or drugs in different classes are used. However, the existing anti-diabetic drugs is lack of long-term efficacy mainly because their mechanism of action is to increase the sensitivity of target tissues to insulin action or improve insulin-producing activity of pancreas, but these drugs have no targeted effect to the reduced function of the pancreatic β cell, which is the fundamental cause of diabetes.
-
Dipeptidyl peptidase-IV (DPP-IV) is widely present in the body, and is a cell surface protein involved in a variety of biological functions. It can degrade many active enzymes in vivo, such as glucagon like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), neuropeptide, substance P, and chemokines and the like. The deficiency of GLP-1 and GIP is the main cause resulting in type II diabetes (i.e., non-insulin-dependent diabetes). DPP-IV inhibitor is a new generation of anti-diabetic drug. It protects the activity of GLP-1, GIP and the like, stimulates the secretion of insulin, lowers blood glucose level by inhibiting the activity of DPP-IV, and does not cause hypoglycemia, weight gain, edema and other side effects. Its effect for lowering blood glucose level stops when a normal blood glucose level has been reached, and hypoglycemia will not occur. It can be used for a long term, and can repair the function of β-cells.
-
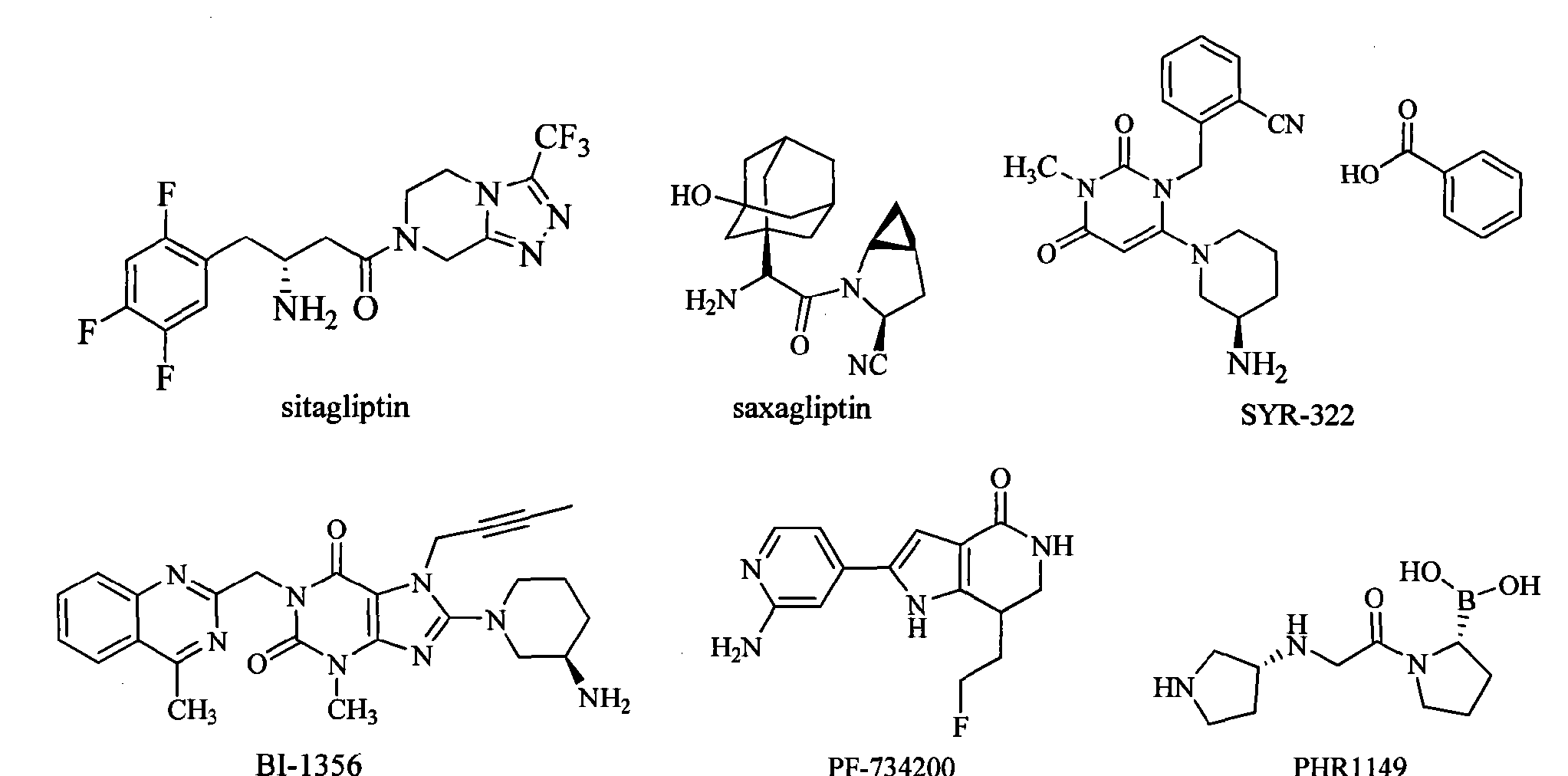
Sitagliptin is the first marketed DPP-IV inhibitor. It rapidly became a “blockbuster” drug after marketed in 2006 by Merck. The FDA approved the saxagliptin developed by AstraZeneca and Bristol-Myers Squibb on July 31, 2009. SYR-322 developed by Takeda has an activity and selectivity better than that of sitagliptin and saxagliptin, and is currently in the phase of pre-registration. In addition, there are three drugs in clinical phase III: BI-1356 (linagliptin) developed by Boehringer Ingelheim, PF-734200 (gosogliptin) developed by Pfizer Inc, and PHX1149 (dutogliptin) developed by Phenomix Inc. Nine drugs are in the clinical phase II, and seven drugs are in clinical phase I.
-
However, the limited varieties of drugs can not satisfy the clinical requirements. Accordingly, there is an urgent need for development of many DPP-IV inhibitor drugs to satisfy the clinical use.
Example 17 The preparation of (R)-2-[[7-(3-aminopiperidin-1-yl)-3,5-dimethyl-2-oxo-2,3-dihydro-1
-imidazo[4,5-b]pyridin-1-yl]methyl]benzonitrile (Compound 17) trifluoroacetate
-
(1)2,4-dichloro-6-methyl-3-nitropyridine
-
-
6-methyl-3-nitropyridin-2,4-diol (1.7 g, 10 mmol) was dissolved in 10 mL POCl3, heated to 95°C, and stirred for 1.5 h. The excess POCl3 was removed through centrifugation. 100 mL ice water was carefully added. The reaction solution was extracted with ethyl acetate (80 mL×3). The organic phase was combined, washed with saturated brine, dried with anhydrous Na2SO4 and spinned to dryness to afford 1.773 g yellow powder with a yield of 85.7 %.
(2) (R)-1-(2-chloro-3-nitro-6-methylpyridin-4-yl)piperidin-3-yl tert-butyl carbamate
-
-
[0216]
The specific operation referred to the step (1) described in Example 1 for details. 0.96 g 2,4-dichloro-6-methyl-3-nitropyridin (4.64 mmol), and 0.933 g R-tert-butylpiperidin-3-yl-carbamate (4.66 mmol) were charged to afford 1.1 g titled product with a yield of 63.9 %.
(3) (R)-1-(2-methylamino-3-nitro-6-methylpyridin-4-yl)piperidin-3-yl tert-butyl carbamate
-
-
The specific operation referred to the step (2) described in Example 1 for details, 1.1 g (R)-1-(2-chloro-3-nitro-6-methylpyridin-4-yl)piperidin-3-yl tert-butyl carbamate (2.97 mmol), and 5 mL 27 % solution of methylamine in alcohol were charged to afford 1.0 g titled product with a yield of 92.1 %.
(4) (R)-1-(2-methylamino-3-amino-6-methylpyridin-4-yl)piperidin-3-yl tert-butyl carbamate
(5)(R)-1-(3,5-dimethyl-2-oxo-2,3-dihydro-1
H
-imidazo[4,5-b]pyridin-7-yl)piperidin-3-yl tert-butyl carbamate
-
-
The specific operation referred to the step (4) described in Example 1 for details. 873 mg (R)-1-(2-methytamino-3-amino-6-methylpyridin-4-yl)piperidin-3-yl tert-butyl carbamate (2.60 mmol), 849 mg triphosgene (2.86 mmol), and 1.39 mL triethylamine (10.4 mmol) were charged to afford 0.813 g titled product with a yield of 86.5 %.
(6)(R)-1-[1-(2-cyanobenzyl)-3,5-dimethyl-2-oxo-2,3-dihydro-1
H
-imidazo[4,5-b] pyridin-7-yl]piperidin-3-yl tert-butyl carbamate
-
-
The specific operation referred to the step (5) described in Example 1 for details.813 mg (R)-1-(3,5-dimethyl-2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-7-yl)piperidin-3-yl tert-butyl carbamate (2.25 mmol), 441 mg 2-(bromomethyl)benzonitrile (2.25 mmol), and 621 mg potassium carbonate (4.50 mmol) were charged to afford 0.757 g titled product with a yield of 70.5%.
(7)(R)-2-[[7-(3-aminopiperidin-1-yl)-3,5-dimethyl-2-oxo-2,3-dibydro-1-imidazo [4,5-b]pyridin-1-yl]methyl]benzonitrile trifluoroacetate
-
-
The specific operation referred to the step (6) described in Example 1 for details. 750 mg (R)-1-[1-(2-cyanobenzyl)-3,5-dimethyl-2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin -7-yl]piperidin-3-yl tert-butyl carbamate (1.57 mmol), and 8.5 mL trifluoroacetic acid were charged to afford 0.680 g titled product with a yield of 88.3%.
Molecular formula: C21H24N6O Molecular weight: 376.45 Mass spectrum (M+H): 377.2
1H-NMR(D2O, 400 MHz): δ 7.64 (d, 1H), 7.42 (t, 1H), 7.29 (d, 1H), 6.93(d, 1H), 6.76(s, 1H), 5.39(d, 1H), 5.25(d, 1H), 3.27(s, 3H), 3.04(m, 1H), 2.90(m, 2H), 2.80-2.60 (m, 2H), 2.48 (m, 1H), 2.32 (s, 3H), 1.90 (m, 1H), 1.54 (m, 1H), 1.32 (m, 1H).
…………………….
PAPER
We report our discovery of a novel series of potent and selective dipeptidyl peptidase IV (DPP-4) inhibitors. Starting from a lead identified by scaffold-hopping approach, our discovery and development efforts were focused on exploring structure–activity relationships, optimizing pharmacokinetic profile, improving in vitro and in vivo efficacy, and evaluating safety profile. The selected candidate, Imigliptin, is now undergoing clinical trial.
Discovery of Imigliptin, a Novel Selective DPP-4 Inhibitor for the Treatment of Type 2 Diabetes
Chutian Shu †,
Hu Ge ‡,
Michael Song †,
Jyun-hong Chen †,
Huimin Zhou †,
Qu Qi †,
Feng Wang †,
Xifeng Ma †,
Xiaolei Yang †,
Genyan Zhang †,
Yanwei Ding †,
Dapeng Zhou †,
Peng Peng †,
Cheng-kon Shih †‡,
Jun Xu ‡, and
Frank Wu *†‡
† Department of Project Management, Medicinal Chemistry, Process, Pharmacology, Drug Metabolism and Pharmacokenetics, Toxicology, XuanZhu Pharma, 2518 Tianchen Street, Jinan, Shandong, The People’s Republic of China
‡ School of Pharmaceutical Sciences & Institute of Human Virology, Sun Yat-Sen University, 132 East Circle Road at University City, Guangzhou, The People’s Republic of China
ACS Med. Chem. Lett., Article ASAP
DOI: 10.1021/ml5001905
http://pubs.acs.org/doi/abs/10.1021/ml5001905
synthesis………http://pubs.acs.org/doi/suppl/10.1021/ml5001905/suppl_file/ml5001905_si_001.pdf
data for LEAD compd 1
mono-TFA solvate (160mg, 71%).
1H NMR (d-DMSO + D2O, 600 MHz):
δ
8.01 (d, 1 H), 7.89 (d, 1 H), 7.69 (t, 1 H),
7.53 (t, 1 H), 7.40 (d, 1 H), 7.13 (d, 1 H),
5.41 (d, 1 H), 5.30 (d, 1 H), 3.25 (d, 1 H), 3.05
(m, 1 H), 2.93 (d, 1 H), 2.77 (m, 1 H),
2.65 (m, 1H), 1.95 (m, 1 H), 1.66 (m, 1 H),
1.46-1.26 (m, 2 H).
Molecular Formula C19H19N5O2:(M+H) 350.2
compd 27

mono-TFA solvate (680 mg, 88%).1H NMR (D2O, 400 MHz):δ7.64 (d, 1 H), 7.42 (t, 1 H), 7.29 (d, 1 H), 6.93(d, 1 H),
6.76 (s, 1 H), 5.39 (d, 1 H), 5.25 (d, 1 H), 3.27(s, 3 H), 3.04 (m, 1 H), 2.90 (m, 2 H),
2.80-2.60 (m, 2 H), 2.48 (m, 1 H), 2.32 (s, 3 H), 1.90 (m, 1 H), 1.54 (m, 1 H), 1.32 (m,1 H).
Molecular Formula C21H24N6O: (M+H) 377.2.
……………………………………………………………………………………….
http://www.sihuanpharm.com/index.php?a=show&m=Article&id=403&l=en
Start of the first 4 volunteers in Imigliptin Dihydrochloride Phase I clinical trial
2013-10-18 16:31:08 Copyfrom: Sihuan Pharmaceutical Holdings Group Ltd.
Sihuan R&D clinical research centre (based in Beijing) announced that four healthy volunteers (human subjects) were administrated Imigliptin Dihydrochloride at first dosage of 5mg this morning around 8:00 am on 18 Oct 2013, and they all are in good conditions without any observed adverse effects so far.This is the first category 1.1 innovative drug independently developed by Sihuan Group which has now officially entered into clinical trials; that is from laboratory research into human studies. The preclinical studies of Imigliptin Dihydrochloride, a novel DPP-4 inhibitor treating type II diabetes, demonstrate excellent in vitro and in vivo activities and selectivities. In animal studies, it can protect pancreatic β–cells in long-term treatment. Pharmacokinetic studies of Imigliptin Dihydrochloride show attractive profile of good oral bioavailability, fast absorption and onset, and longer half-life compatible with the once daily dosing. We anticipate the above mentioned preclinical profiles be confirmed in our ongoing clinical trials.
………………………..
http://www.google.com/patents/CN102127072A?cl=en
Sitagliptin (sitagliptin) is the first one listed on the DPP-IV inhibitor, in 2006 after the listing quickly became a blockbuster for Merck. July 31, 2009, FDA has approved AstraZeneca and Bristol-Myers Squibb developed saxagliptin (saxagliptin) listed. Takeda (Taketa)’s SYR-322 activity and selectivity are superior to sitagliptin and saxagliptin, is currently in pre-registration. In addition, there are three stages of drug is in phase III: Bo Mingge Yan Gehan’s BI-1356 (Iinagliptin), Pfizer’s PF-734200 (gosogliptin), phenomix company PHX 1149 (dutogliptin) [0007]
In phase II drug has nine, in phase I of seven.
[0008] However, the limited varieties of drugs, can not meet the clinical needs, the urgent need to develop more of the DPP-IV inhibitor drugs to meet the clinical medication.
Example 17 (R)-2-ΓΓ7-(3 ~ amino-piperidin-yl) -3, 5_ dimethyl _2_ oxo, 3_ dihydro-IH-blind half and P “4,5 Pyridine-b1-i-a] benzonitrile Jiamou 1 (Compound 17) The system of the
[0451]
[0452] (1) 2,4 – dichloro-6 – methyl-nitropyridine _3_
[0453]
[0454] A mixture of 6 – methyl-3 – nitropyridine 2,4 – diol (1. Lg, IOmmol) dissolved in IOmL POCl3, heated to 95 ° C, stirred for 1.5 hours, rotating to excess POCl3 , ice water was added carefully IOOmL, extracted with ethyl acetate (80mLX3), the combined organic phases washed with saturated brine, dried over anhydrous Na2SO4, rotary done 1. 773g yellow powder, yield 85.7%.
[0455] (2) (R)-I-(2 – chloro-nitro _6_ _3_ _4_ picoline) piperidin-_3_ t-butyl carbamate
[0456]
[0457] Specific operation in Reference Example 1 (1), cast _ 2,4 dichloro-6 – methyl-_3_ nitropyridine 0. 96g (4. 64mmol), R-tert-butyl piperidin-_3_ yl – carbamate 0. 933g (4. 66mmol), to give the product 1. Ig, yield 63.9%.
[0458] (3) (R)-I-(2 – methylamino-nitro _6_ _3_ _4_ picoline) piperidin-_3_ t-butyl carbamate
[0459]
[0460] Specific operation in Reference Example 1 (2), cast (R) -1 – (2 – chloro-nitro _6_ picoline _3_ _4_ yl)-piperidin-3 – tert-butyl imino ester 1. Ig (2. 97mmol), 27% methylamine alcohol solution 5mL, to give the product 1. Og, yield 92.1%.
[0461] (4) (R)-I-(2 – methyl amino -3 – diamino-6 – methylpyridine _4_ yl) piperidin-_3_ t-butyl carbamate
[0462]
[0463] Specific operation in Reference Example 1 (3), cast (R)-l_ (2 – methylamino-methyl-4 _3_ nitro _6_ – yl) piperidin-3 – tert- butyl carbamate 1.0g (2. 74mmol), 10% Pd-C 0. lg, to give the product 0. 873g, 95% yield.
[0464] (5) (R)-I-(3,5 – dimethyl-2 – oxo-2 ,3 – dihydro-IH-imidazo [4,5 _b] pyridin _7_ yl)
Piperidin-3 – t-butyl carbamate
[0465]
[0466] Specific operation in Reference Example 1 (4), cast ((R)-l_ (2 – methylamino-4 _3_ methyl amino _6_ – yl) piperidin-3 – yl t-butyl carbamate 873mg (2. 60mmol), triphosgene 849mg (2. 86mmol), triethylamine 1. 39mL (10. 4mmol), to give the product 0. 813g, yield 86.5% 0
[0467] (6) (R)-l-[l_ (2 – cyano-benzyl) -3,5 _ dimethyl-2 – oxo-2 ,3 – dihydro-IH-imidazo [4, 5 -b] pyridin-7 – yl] piperidin-3 – t-butyl carbamate
[0468]
[0469] Specific operation in Reference Example 1 (5), cast (R)-I-(3,5 – dimethyl-2 – oxo-2 ,3 – dihydro-IH-imidazo [4, 5-b] pyridin-7 – yl) piperidin-3 – t-butyl carbamate 813mg (2. 25mmol), 2_ (bromomethyl) benzonitrile 441mg (2. 25mmol), potassium carbonate 621mg (4. 50mmol), to give the product 0. 757g, yield 70.5%.
[0470] (7) (R) -2 – [[7 – (3 – amino-piperidin-1 – yl) -3,5 – dimethyl-2 – oxo-2 ,3 – dihydro-IH- imidazo [4,5-b] pyridin-1 – yl] methyl] benzonitrile
[0471]
[0472] Specific operation in Reference Example 1 (6), cast (R)-l-[l_ (2 – cyano-benzyl) -3,5-dimethyl-2-_ – oxo – two H-IH-imidazo [4,5-b] pyridin-7 – yl] piperidin-3 – t-butyl carbamate 750mg (l. 57mmol), trifluoroacetic acid 8. 5mL, 0 to give the product . 680g, yield 88.3%.
[0473] MF = C21H24N6O MW: 376 * 45 MS (M + H): 377. 2
[0474] 1H-NMR (D2OdOOMHz): δ 1. 32 (1Η, m), 1. 54 (1H, m), 1. 90 (1H, m), 2. 32 (3H, s), 2. 48 (1H, m), 2. 80-2. 60 (m, 2H), 2. 90 (2H, m), 3. 04 (1H, m), 3. 27 (3H, s), 5. 25 ( 1H, d), 5. 39 (1H, d), 6. 76 (1H, s), 6. 93 (1H, d), 7. 29 (1H, d), 7. 42 (1H, t), 7. 64 (1H, d) ·
| WO2004050658A1 * |
Dec 3, 2003 |
Jun 17, 2004 |
Boehringer Ingelheim Pharma |
Novel substituted imidazo-pyridinones and imidazo-pyridazeiones, the production and use thereof as medicaments |
| WO2009099594A1 * |
Feb 2, 2009 |
Aug 13, 2009 |
Luke W Ashcraft |
Certain chemical entities, compositions and methods |
| WO2011085643A1 * |
Jan 17, 2011 |
Jul 21, 2011 |
Kbp Biomedical Co., Ltd. |
Fused pyridine derivatives |
| CN101228164A * |
May 11, 2006 |
Jul 23, 2008 |
布里斯托尔-迈尔斯·斯奎布公司 |
Pyrrolopyridine-based inhibitors of dipeptidyl peptidase IV and methods |

 Ghent Research Group on Nanomedicine, Ghent University, B9000 Ghent,Belgium
Ghent Research Group on Nanomedicine, Ghent University, B9000 Ghent,Belgium







































 R1 = NEOPENTYL R2=H
R1 = NEOPENTYL R2=H

















 BRAZIL WORLDCUP WEEK 2014
BRAZIL WORLDCUP WEEK 2014









