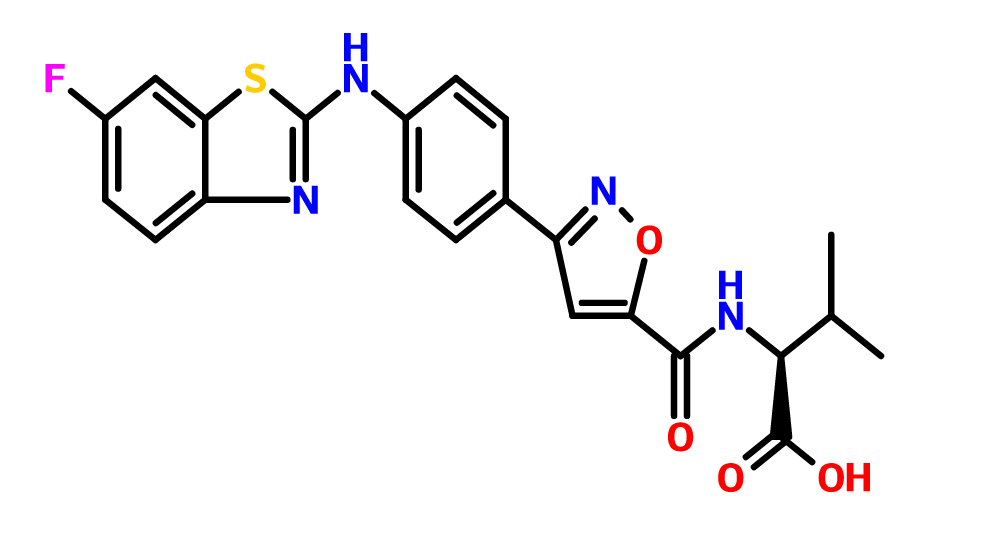
Piramal Enterprises Limited announced that its wholly owned subsidiary in the U.S. has entered into an agreement to acquire 100 percent stake in Ash Stevens Inc., a U.S.-based contract development and manufacturing organization (CDMO), in an all cash deal for a consideration of USD $42.95 million plus an earn-out consideration capped at $10 million. This potential transaction is expected to be completed by the end of August.
Located in Riverview, Michigan, Ash Stevens has over 50 years of experience in contract manufacturing, and serves several biotech, mid-size pharma, and large pharmaceutical clients worldwide.
With over 60,000 sq. ft. of facilities, eight chemical drug development and production laboratories, and six full-scale production areas, Ash Stevens has built a stellar reputation, led by science, driven by operational excellence, and one that emphasizes quality as a culture. As one of the leaders in HPAPI manufacture, Ash Stevens has an impeccable safety record of working with high potency anti-cancer agents and other highly-potent therapeutics. The state-of-the-art manufacturing facility in Michigan features all necessary engineering and containment controls for the safe handling and cGMP manufacture of small and large-scale HPAPIs, with Occupational Exposure Limits (OELs) ≤ 0.1µg/m3. The facility has approvals from U.S., EU, Australia, Japan, Korea, and Mexico regulatory agencies.
“The acquisition of Ash Stevens fits well with our strategy to build an asset platform that offers value to our partners and collaborators. Currently, around 25 percent of the molecules in clinical development are potent. Our clients are looking for reliable partners that can assist them in advancing these programs forward,” said Vivek Sharma, CEO of Piramal Pharma Solutions. He further adds, “North America is a key market that we can now service with our three local facilities – the Coldstream Labs in Kentucky for fill finish needs, the Torcan facility in Toronto for complex high value APIs and now, Ash Stevens in Michigan for HPAPIs. Having facilities with a differentiated platform and geographical proximity to clients are keys towards building strategic partnerships. We expect this acquisition to also be synergistic with our Antibody Drug Conjugates (ADCs) and injectable business. We can now fulfill client requirements for a single source of supply for both high potent APIs and drug products.”
“With its rich history of scientific excellence, a track record of 12 product launches, Ash Stevens is well poised to become the partner of choice for clients looking to advance programs from early development through launch. In addition to the business benefits that the combined entity will bring to our clients, I am also pleased that the firms share common core values: both were founded by successful entrepreneurs, value integrity, and are committed to a customer-first approach,” said Dr. Mark Cassidy, President of the API Business at Piramal Pharma Solutions. “I am pleased to welcome the Ash Stevens team into the Piramal group. We expect them to be an integral part of our future growth plans.”
Added Dr. Stephen Munk, CEO of Ash Stevens, “We look forward to working with the Piramal leadership and management team, to develop API solutions that benefit customers and improve the lives of patients. The commitment that Piramal has shown towards growing its healthcare businesses, coupled with the complementary capabilities that our two firms have, makes this an exciting time for Ash Stevens and our employees. We have already identified areas where we can create significant value together, and will be moving forward rapidly to achieve those objectives.”
The transaction is not subject to any regulatory approvals. No related party of PEL has any interest in Ash Stevens.
Wells Fargo Securities, LLC served as exclusive financial advisor to Ash Stevens, with legal counsel provided by Morrison & Foerster LLP.
For further information on the financials, please visit our website: www.piramal.com.
Ajay Piramal

////////////CDMO, Ash Stevens, Piramal Enterprises, Stephen A. Munk