Green Chem., 2017, 19,5163-5171
DOI: 10.1039/C7GC02190A, Paper
David K. Leahy, Eric M. Simmons, Victor Hung, Jason T. Sweeney, William F. Fleming, Melanie Miller
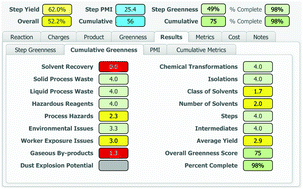
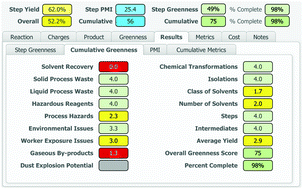
A process greenness scorecard has been developed that provides a comprehensive assessment of greenness aspects not encompassed by mass-based metrics, including environmental, health and safety impacts, in order to facilitate the design of greener, more benign and inherently safer processes.
The content of this RSS Feed (c) The Royal Society of Chemistry

Melanie Miller
Executive Director, Pharmaceutical Development at Bristol Myers Squibb
Head of API Operations, Pharmaceutical Development
Bristol Myers Squibb
New Brunswick, New Jersey
Design and evolution of the BMS process greenness scorecard
*Corresponding authors
aChemical and Synthetic Development, Bristol-Myers Squibb, New Brunswick, USA
E-mail:eric.simmons@bms.com
bAPI Operations, Bristol-Myers Squibb, New Brunswick, USA
cDrug Product Science and Technology, Bristol-Myers Squibb, New Brunswick, USA
dEnvironmental Health Safety and Sustainability, Bristol-Myers Squibb, East Syracuse, USA

Jason Sweeney
Associate Director at Bristol-Myers Squibb
Abstract
An accurate and comprehensive assessment of the environmental, health and safety impacts of a chemical process is critical to the design and implementation of greener, more benign and inherently safer processes. Over the past 15 years at BMS, we have developed a Process Greenness Scorecard to capture and analyse a number of metrics and attributes for each step in the synthetic sequence used to produce an API. This manuscript describes the design and evolution of the scoring methodology and implementation of the resulting scorecard, from an initial Excel-based tool to the current web-based format.
-

Associate Director, Process Chemistry
Company NameTakeda
Dates EmployedJul 2017 – Present
Employment Duration4 mos
LocationCambridge, MA
-
Principal Scientist
Company NameBristol-Myers Squibb
Dates Employed2013 – 2017
Employment Duration4 yrs
LocationGreater New York City Area
Introduction
“The ability to meet the needs of the present without compromising the ability of future generations to meet their needs” The definition of sustainable development from the United Nations World Commission on Environment and Development has indeed resonated with corporate leaders across the globe.1 This is evident by the wealth of public-facing sustainability goals that Fortune 500 companies have committed to over the last decade. Within the context of the pharmaceutical industry, it is green chemistry2 that provides the key to environmentally-responsible pharmaceutical manufacturing3 , and practitioners have been rewarded with enormous impacts to their triple bottom line.4
Green chemistry is more cost-effective, safer for employees, and better for the environment. Corporate sustainability has been characterized as a key driver for innovation, which is essential for a firm to succeed.5 As part of our program in green chemistry, we anticipated that a tool that could assess the ‘greenness’ of our chemical processes would spark the innovation of our scientists, by pointing out deficient areas, prompting focus on these areas, and providing quantitative evidence that their improvements had the desired impact.6
A number of mass-based metrics are available to assess the greenness of a chemical process,7,8 with E factor (kg of waste/kg of product)9 and Process Mass Intensity (PMI = kg of inputs/kg of product)10 being most widely utilized within the pharmaceutical industry.7,8,10 We firmly believe that such metrics are very important, but we also recognized that they ignore many key green chemistry principles, most importantly safety.7,11,12
While important strides have been made in the development of quantitative methods to compare the environmental impact of chemical syntheses,13 most notably though Life Cycle Assessment (LCA)14 and the FLASC tool,15 as well as the recently introduced Green Aspiration Level (GAL),7,12 metrics that assess the safety and health hazards of chemical processes and products are lacking in comparison.16,17
In this article, we describe the strategy we have taken at Bristol-Myers Squibb to expand on existing mass-based approaches to include a comprehensive assessment of the important facets of greenness not encompassed by typical process metrics, such as E factor and PMI, to develop a Process Greenness scoring methodology that is appropriate for the assessment of the chemical processes used on scale for the synthesis of smallmolecule active pharmaceutical ingredients (APIs) and intermediates.18,19

Eric Simmons
Senior Research Investigator II at Bristol-Myers Squibb
Conclusions
The BMS process greenness scorecard is an important tool for scientists to help guide decisions made during API process development. It serves as the key methodology we use to assess the environmental and safety performance of our processes to manufacture compounds in development. This greenness score provides a useful and quantitative method, complimentary to mass based metrics such as PMI and derived from the 12 principles of green chemistry. A key advantage of this assessment is that it also considers the inherent safety of a process, both from a worker exposure and process hazards perspective. These are key green chemistry considerations that are not captured when evaluating a process using mass-based metrics alone. This assessment is especially important when facing complex decisions involving tradeoffs between improved efficiency versus enhanced process safety. While this tool is currently only suitable for use in evaluating small molecules, efforts are underway to expand this methodology to assess other important therapeutic modalities, including synthetic peptides, oligonucleotides, antibody-drug conjugates, and biologics and will be reported in due course.

William Fleming
Director, Head of Safety, Global EHS&S
Bristol-Myers Squibb
Chemical and Synthetic Development, Bristol-Myers Squibb, New Brunswick, USA
////////////////Bristol-Myers Squibb, bms, green
ref http://pubs.rsc.org/en/Content/ArticleLanding/2017/GC/C7GC02190A?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
more…………..
DOI:
10.1039/C6GC02359B (Paper)
Green Chem., 2017,
19, 127-139
A data-driven strategy for predicting greenness scores, rationally comparing synthetic routes and benchmarking PMI outcomes for the synthesis of molecules in the pharmaceutical industry†
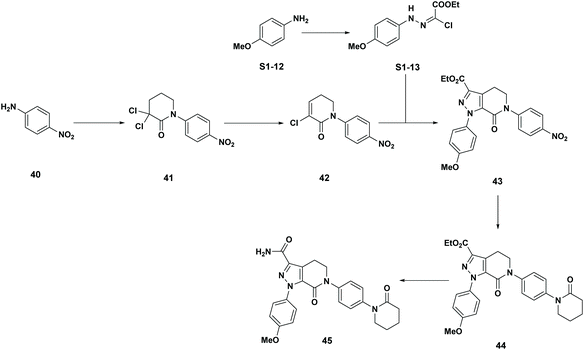
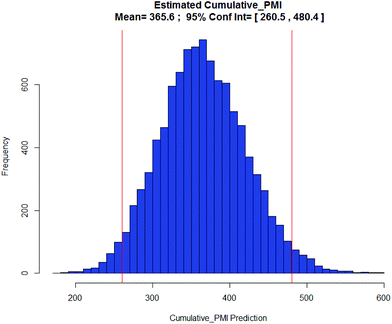
Apixaban: Our final case study is apixaban (45), an orally bioavailable inhibitor of blood coagulation factor Xa, developed for thrombotic diseases and commercialized as Eliquis (Scheme 6).17 This highly optimized process evolved through multiple rounds of development and the data reported is taken from the validation campaign, thus ready for product launch. The actual cumulative PMI for the overall process was 197, which is significantly below the lower end of the 95% confidence interval for the predicted cumulative PMI (Fig. 14). In essence, it is lower than 99.9% of the similar chemistries executed on scale at different development stages. This is the one of a few commercial assets in our current database, and while obviously efficient, this score should be viewed with the perspective that most of the data available to us in this proof of concept study is in the development phase, and thus encompasses a wide range of optimization levels. However, in order to compare more globally, more data, from more companies, and across all phases of development is needed.
 |
|
Scheme 6 Apixaban synthetic route in validation campaign. |
|
Fig. 14 Predicted apixaban cumulative PMI with mean 366 and 95% CI between 261 and 480. |
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent

















 Associate Director, Process Chemistry
Associate Director, Process Chemistry