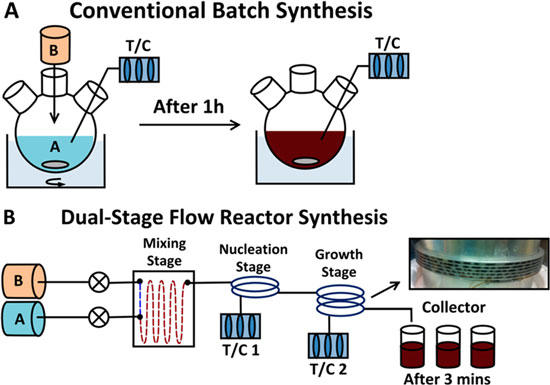
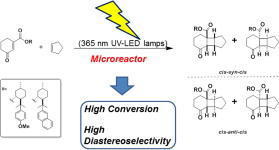
The diastereoselective [2+2] photocycloaddition of ethylene to a chiral cyclohexenone was studied in a continuous flow microcapillary reactor. In all cases examined, the microcapillary reactor gave higher conversions and selectivity than the batch system, even after shorter irradiation times. These findings were explained by the superior temperature control, favorable light penetration, and generation of a gas–liquid slug flow with improved mass transfer in the microreactor.
http://www.akademiai.com/content/03163u0p80225v14/?p=bb18d4ec7c044f5c80013806493e8850&pi=2
Authors
Kimitada Terao
1, Yasuhiro Nishiyama
1, Hiroki Tanimoto
1, Tsumoru Morimoto
1, Michael Oelgemöller
2, Kiyomi Kakiuchi
1 
kakiuchi@ms.naist.jp, http://mswebs.naist.jp/LABs/kakiuchi/member/staff/CV_kakiuchi.pdf
1Nara Institute of Science and Technology (NAIST) Graduate School of Materials Science 8916-5 Takayama-cho, Ikoma Nara 630-0192 Japan
2James Cook University School of Pharmacy and Molecular Sciences Townsville QLD 4811 Australia
more………..
http://mswebs.naist.jp/LABs/kakiuchi/achevement/paper.htm
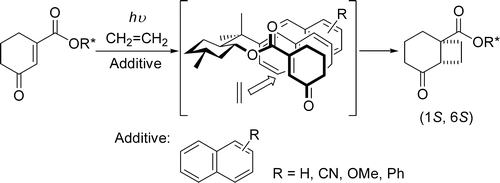
“Novel Enhancement of Diastereoselectivity of [2+2] Photocycloaddition of
Chiral Cyclohexenones to Ethylene by Adding Naphthalenes”
Ken Tsutsumi, Hiroaki Nakano, Akinori Furutani, Katsunori Endou, Abdurshit Merpuge
Takuya Shintani, Tsumoru Morimoto, Kiyomi Kakiuchi
J. Org. Chem. 2004, 69, 3, 785-789.
“Diastereoselective [2+2] Photocycloaddition of Polymer-Supported
Cyclic Chiral Enone with Ethylene”
Takuya Shintani, Kazunori Kusabiraki, Atsuko Hattori, Akinori Furutani, Ken Tsutsumi,
Tsumoru Morimoto, Kiyomi Kakiuchi
Tetrahedron Lett. 2004, 45, 9, 1849-1851.
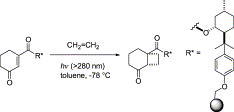
“Diastereoselective [2+2] Photocycloaddition of Cyclohexenone Derivative with Olefines in Supercritical Carbon Dioxide”
Yasuhiro Nishiyama, Kazuya Nakatani, Hiroki Tanimoto, Tsumoru Morimoto, Kiyomi Kakiuchi
J. Org. Chem. 2013, 78, 7186-7193.
Highlighted in
ChemInform 2013, 44(44)
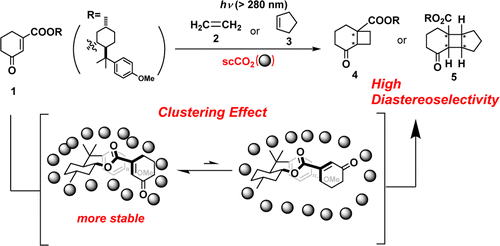
“Diastereoselective [2+2] Photocycloaddition of Chiral Cyclic Enones with Olefins in Aqueous Media Using Surfactants”
Yasuhiro Nishiyama, Mikiko Shibata, Takuya Ishii, Tsumoru Morimoto, Hiroki Tanimoto,
Ken Tsutsumi, Kiyomi Kakiuchi
Molecules, 2013, 18, 1626-1637.
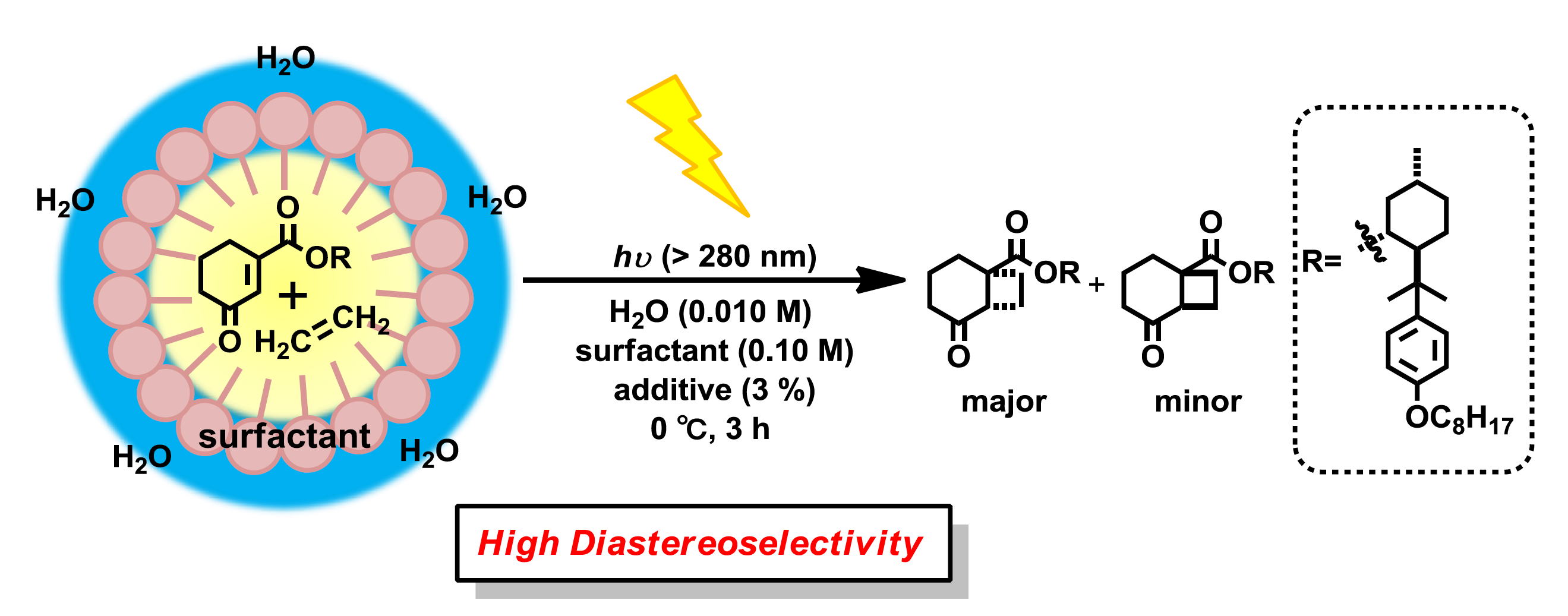
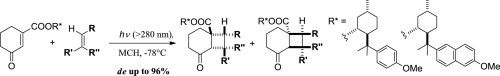
“Highly diastereodifferentiating and regioselective [2+2]-photoreactions using methoxyaromatic menthyl cyclohexenone carboxylates”
Inga Inhulsen, Naoya Akiyama, Ken Tsutsumi, Yasuhiro Nishiyama, Kiyomi Kakiuchi
Tetrahedron 2013, 69, 782-790.
“Diastereodifferentiating [2+2] Photocycloaddition of Chiral Cyclohexenone Carboxylates with Cyclopentene by a Microreactor”
Kimitada Terao, Yasuhiro Nishiyama, Shin Aida, Hiroki Tanimoto, Tsumoru Morimoto,
Kiyomi Kakiuchi
J. Photochem. Photobiol. A: Chem. 2012, 242, 13-19.


 Charlotte
Charlotte