Styryl Hydrazine Thiazole Hybrids
Will be updated………kindly email amcrasto@gmail.com
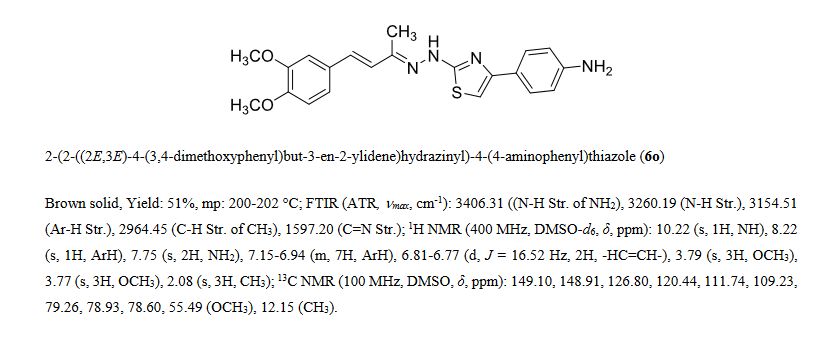
DATA
ABOUT Dehydrozingerone
Dehydrozingerone; Feruloylmethane; 1080-12-2; 4-(4-Hydroxy-3-methoxyphenyl)-3-buten-2-one; 4-(4-hydroxy-3-methoxyphenyl)but-3-en-2-one; Vanillalacetone;
http://pubs.acs.org/doi/abs/10.1021/np300465f

Dehydrozingerone (1) is a pungent constituent present in the rhizomes of ginger (Zingiber officinale) and belongs structurally to the vanillyl ketone class. It is a representative of half the chemical structure of curcumin (2), which is an antioxidative yellow pigment obtained from the rhizomes of turmeric (Curcuma longa). Numerous studies have suggested that 2 is a promising phytochemical for the inhibition of malignant tumors, including colon cancer. On the other hand, there have been few studies on the potential antineoplastic properties of 1, and its mode of action based on a molecular mechanism is little known. Therefore, the antiproliferative effects of1 were evaluated against HT-29 human colon cancer cells, and it was found that 1 dose-dependently inhibited growth at the G2/M phase with up-regulation of p21. Dehydrozingerone additionally led to the accumulation of intracellular ROS, although most radical scavengers could not clearly repress the cell-cycle arrest at the G2/M phase. Furthermore, two synthetic isomers of1 (iso-dehydrozingerone, 3, and ortho-dehydrozingerone, 4) were also examined. On comparing of their activities, accumulation of intracellular ROS was found to be interrelated with growth-inhibitory effects. These results suggest that analogues of 1 may be potential chemotherapeutic agents for colon cancer
PAPER

Series of styryl hydrazine thiazole hybrids inspired from dehydrozingerone (DZG) scaffold were designed and synthesized by molecular hybridization approach. In vitro antimycobacterial activity of synthesized compounds was evaluated against Mycobacterium tuberculosis H37Rv strain. Among the series, compound 6o exhibited significant activity (MIC = 1.5 μM; IC50 = 0.48 μM) along with bactericidal (MBC = 12 μM) and intracellular antimycobacterial activities (IC50 = <0.098 μM). Furthermore, 6o displayed prominent antimycobacterial activity under hypoxic (MIC = 46 μM) and normal oxygen (MIC = 0.28 μM) conditions along with antimycobacterial efficiency against isoniazid (MIC = 3.2 μM for INH-R1; 1.5 μM for INH-R2) and rifampicin (MIC = 2.2 μM for RIF-R1; 6.3 μM for RIF-R2) resistant strains of Mtb. Presence of electron donating groups on the phenyl ring of thiazole moiety had positive correlation for biological activity, suggesting the importance of molecular hybridization approach for the development of newer DZG clubbed hydrazine thiazole hybrids as potential antimycobacterial agents.
Dehydrozingerone Inspired Styryl Hydrazine Thiazole Hybrids as Promising Class of Antimycobacterial Agents
///////Antimycobacterial activity, bactericidal, dehydrozingerone, NIAID, thiazole, PRECLINCAL
c1(ccc(c(c1)OC)OC)/C=C/C(C)=N/Nc2nc(cs2)c3ccc(cc3)N