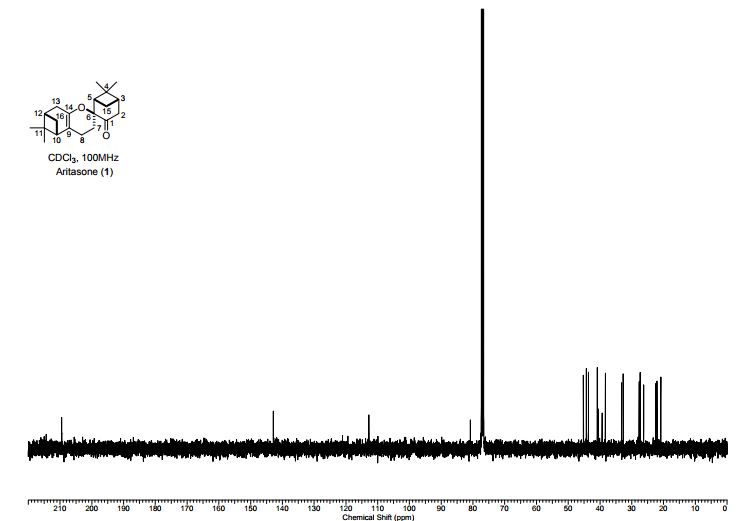
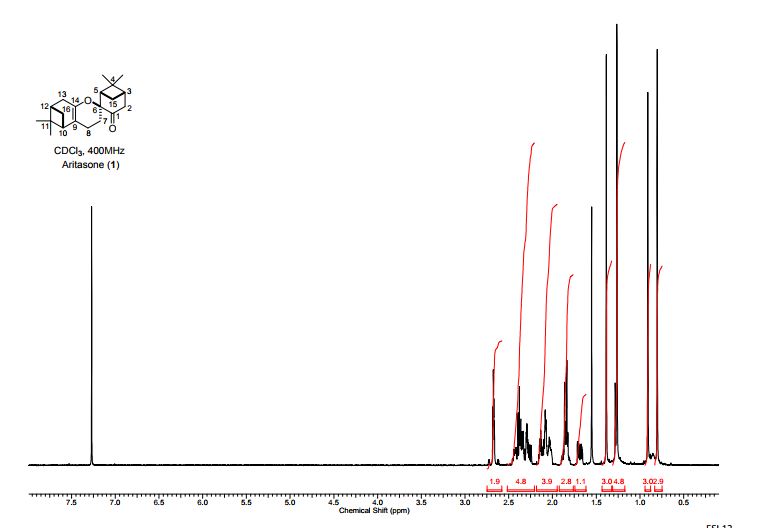
Total synthesis of (-)-aritasone via the ultra-high pressure hetero-Diels-Alder dimerisation of (-)-pinocarvone
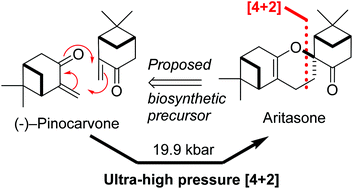
The total synthesis of aritasone via the proposed biosyntheic hetero-Diels-Alder [4 + 2] cyclodimerisation of pinocarvove, has been achieved under ultra-high pressure (19.9 kbar) conditions
Total synthesis of (−)-aritasone via the ultra-high pressure hetero-Diels–Alder dimerisation of (−)-pinocarvone
Abstract
This paper describes a total synthesis of the terpene-derived natural product aritasone via the hetero-Diels–Alder [4 + 2] cyclodimerisation of pinocarvove, which represents the proposed biosyntheic route. The hetero-Diels–Alder dimerisation of pinocarvone did not proceed under standard conditions, and ultra-high pressure (19.9 kbar) was required. As it seems unlikely that these ultra-high pressures are accessible within a plant cell, we suggest that the original biosynthetic hypothesis be reconsidered, and alternatives are discussed.
Christopher Hayes
Contact
- Room C16 School of Chemistry
University Park
Nottingham
NG7 2RD
UK - 0115 951 3045
- 0115 951 3564
- chris.hayes@nottingham.ac.uk
- http://www.nottingham.ac.uk/~pczcjh/Homepage.html
Biography
Prof. Christopher Hayes began his academic career here in Nottingham with his B.Sc. in July 1992. Remaining at Nottingham, he completed his Ph.D. studies in organic chemistry, under the supervision of Professor Gerald Pattenden, in September 1995. In January 1996, on a NATO Postdoctoral Fellowship, he moved to the University of California at Berkeley where he worked in the group of Professor Clayton H. Heathcock. In September 1997, he returned to Nottingham as a Lecturer in Organic Chemistry, and has subsequently been promoted to Reader (2003), Associate Professor (2006) and Professor of Organic Chemistry (2011).
Research Summary
Research is centred in main-stream synthetic organic chemistry, focusing on the organic chemistry of biologically active molecules. His current research interests span a number of areas such as (i)… read more
Recent Publications
-
NICOLLE, SIMON M., LEWIS, WILLIAM, HAYES, CHRISTOPHER J. and MOODY, CHRISTOPHER J., 2016. Stereoselective Synthesis of Functionalized Pyrrolidines by the Diverted N-H Insertion Reaction of Metallocarbenes with -Aminoketone Derivatives: Angewandte Chemie-International Edition Angewandte Chemie-International Edition. 55(11), 3749-3753
-
BARTON, NAOMI A., MARSH, BENJAMIN J., LEWIS, WILLIAM, NARRAIDOO, NATHALIE, SEYMOUR, GRAHAM B., FRAY, RUPERT and HAYES, CHRISTOPHER J., 2016. Accessing low-oxidation state taxanes: is taxadiene-4(5)-epoxide on the taxol biosynthetic pathway?: Chemical Science Chemical Science. 7(5), 3102-3107
-
PALFRAMAN, MATTHEW J., ALHARTHY, RIMA D., POWALOWSKA, PAULINA K. and HAYES, CHRISTOPHER J., 2016. Synthesis of triazole-linked morpholino oligonucleotides via Cu-I catalysed cycloaddition: Organic & Biomolecular Chemistry Organic & Biomolecular Chemistry. 14(11), 3112-3119
-
NICOLLE, SIMON M., HAYES, CHRISTOPHER J. and MOODY, CHRISTOPHER J., 2015. Alkyl Halide-Free Heteroatom Alkylation and Epoxidation Facilitated by a Recyclable Polymer-Supported Oxidant for the In-Flow Preparation of Diazo Compounds: Chemistry-a European Journal Chemistry-a European Journal. 21(12), 4576-4579