DOI: 10.1039/C7MD00656J, Research Article
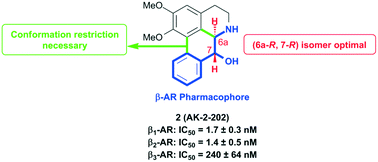
Potent beta-1 and beta-2 adrenergic receptor antagonism via a conformationally restricted aporphine scaffold with defined stereochemistry has been developed.
Discovery of 7-hydroxyaporphines as conformationally restricted ligands for beta-1 and beta-2 adrenergic receptors
Abstract
A series of (−)-nornuciferidine derivatives was synthesized and the non-natural enantiomer of the aporphine alkaloid was discovered to be a potent β1– and β2-adrenergic receptor ligand that antagonized isoproterenol and procaterol induced cyclic AMP increases from adenylyl cyclase, respectively. Progressive deconstruction of the tetracyclic scaffold to less complex cyclic and acyclic analogues revealed that the conformationally restricted (6a-R,7-R)-7-hydroxyaporphine 2 (AK-2-202) was necessary for efficient receptor binding and antagonism.
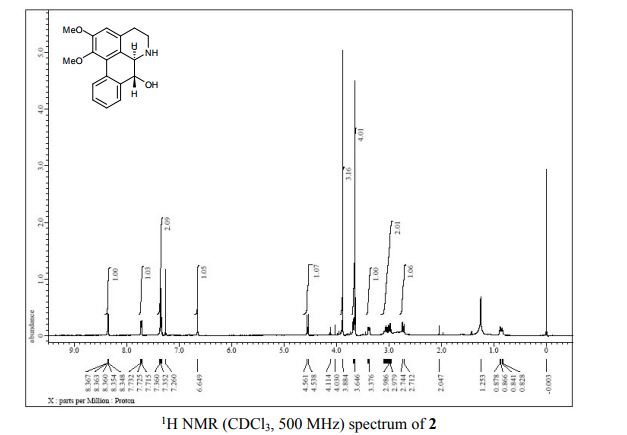
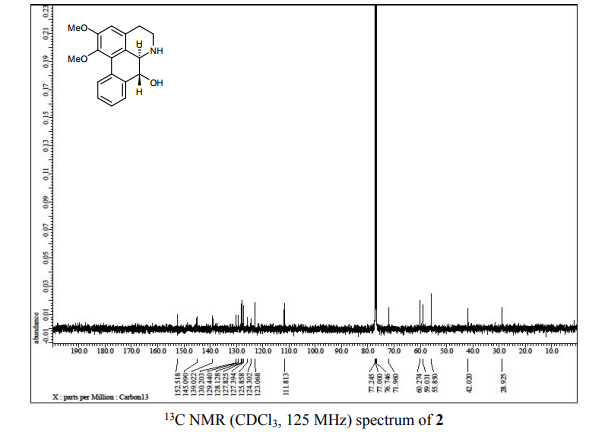
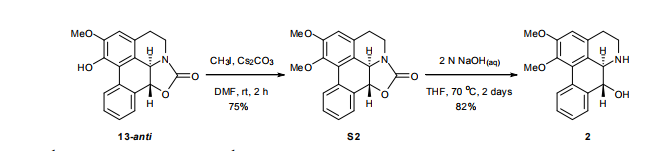
(6aR,7R)-1,2-Dimethoxy-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolin-7-ol (2) To a solution of S2 (10 mg, 0.031 mmol) in THF (2 mL) was added 2 N NaOH(aq) (1 mL), and the mixture was stirred at 70 oC for 2 days. After being quenched with H2O (10 mL), the aqueous layer was extracted with EtOAc (2 × 20 mL). The combined organic extracts were washed with brine, dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (CH3OH/CH2Cl2, 5:95 to 10:90) to afford 2 (7.6 mg, 82%) as a pale yellow solid; mp 89−91 oC; [] 24 D +78 (c 0.58, CHCl3); 1H NMR (CDCl3, 500 MHz) 8.37−8.35 (1 H, m), 7.73−7.72 (1 H, m), 7.38−7.33 (2 H, m), 6.65 (1 H, s), 4.55 (1 H, d, J = 11.5 Hz), 3.88 (3 H, s), 3.67 (1 H, d, J = 11.5 Hz), 3.64 (3 H, s), 3.40−3.37 (1 H, m), 3.10−3.03 (1 H, m), 2.98 (1 H, td, J = 11.5, 3.5 Hz), 2.73 (1 H, d, J = 16.0 Hz); 13C NMR (CDCl3, 125 MHz) 152.5, 145.1, 139.0, 130.2, 129.4, 128.1, 127.8, 127.4, 125.9, 124.3, 123.1, 111.8, 72.0, 60.3, 59.0, 55.9, 42.0, 28.9; HRMS (ESI/Q-TOF) m/z [M + H]+ calculated for C18H20NO3 298.1438; found 298.1440
SIMILAR IN LIT
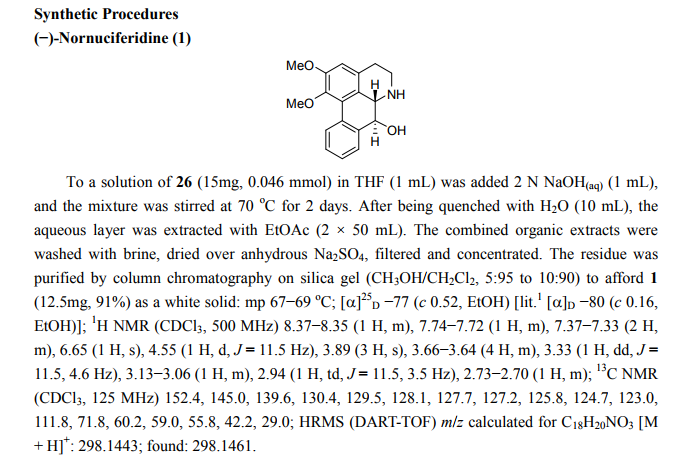
- (-)-Nornuciferidine
-
112494-69-6Molecular Weight297.35, C18 H19 N O34H-Dibenzo[de,g]quinolin-7-ol, 5,6,6a,7-tetrahydro-1,2-dimethoxy-, (6aS-cis)-
- S S ISOMER
- http://pubs.acs.org/doi/suppl/10.1021/acs.orglett.5b00007/suppl_file/ol5b00007_si_001.pdf
-
Synthetic Studies of 7-Oxygenated Aporphine Alkaloids: Preparation of (−)-Oliveroline, (−)-Nornuciferidine, and Derivatives
†Department of Chemistry and ‡Department of Pharmacological and Pharmaceutical Sciences, University of Houston, Science and Research Building 2, Rm 549A, Houston, Texas 77204, United StatesOrg. Lett., 2015, 17 (5), pp 1134–1137DOI: 10.1021/acs.orglett.5b00007*E-mail: gdcuny@central.uh.edu.Abstract
7-Oxygenated aporphines 1–6 possessing anti-configurations have previously been reported. In order to explore their bioactivities, a synthesis was established by utilizing a diastereoselective reductive acid-mediated cyclization followed by palladium-catalyzed ortho-arylations. Moderate XPhos precatalyst loading (10 mol %) and short reaction times (30 min) were sufficient to mediate the arylations. Alkaloids 1–5 were successfully prepared, while (−)-artabonatine A was revised to syn-isomer 30. Consequently, (−)-artabonatine E likely also has a syn-configuration (31).
///////////AK-2-202,