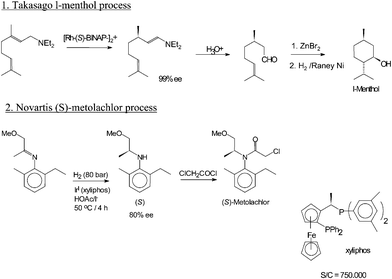
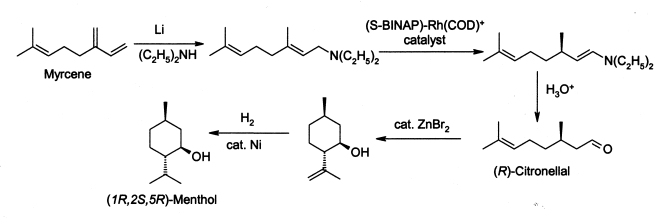
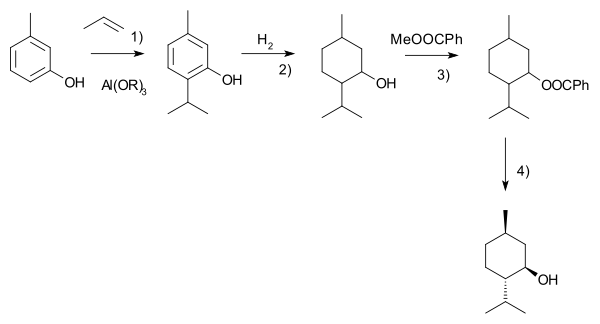
The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride,dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine.The reaction is known for its mild character and wide tolerance of functional groups.

The by-products are dimethyl sulfide (Me2S), carbon monoxide (CO), carbon dioxide (CO2) and — when triethylamine is used as base — triethylammonium chloride (Et3NHCl). Two of the by-products, dimethyl sulfide and carbon monoxide, are very toxic volatile compounds, so the reaction and the work-up needs to be performed in a fume hood.Dimethyl sulfide is a volatile liquid (B.P. 37 °C) with an extremely unpleasant odour.
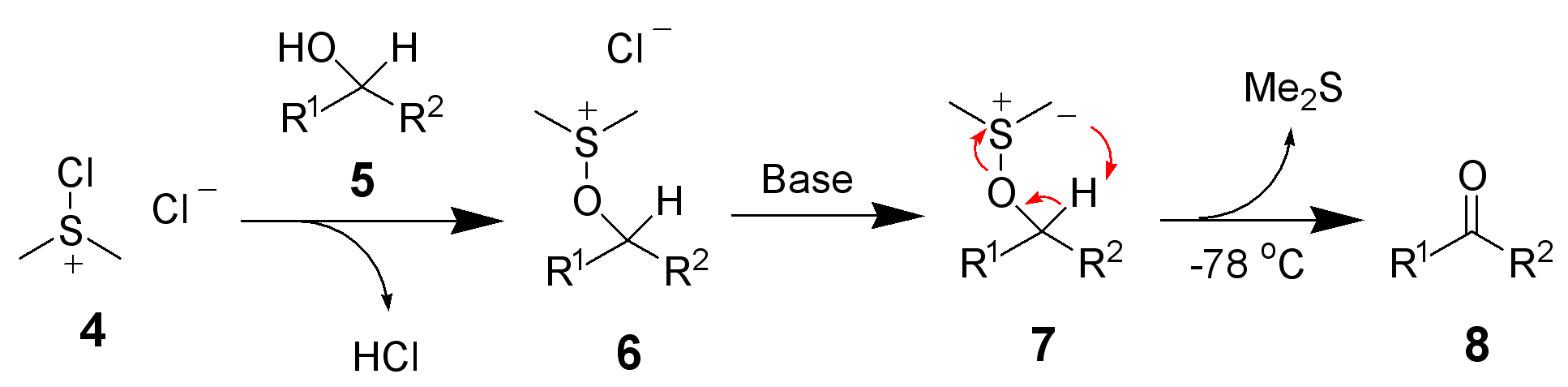
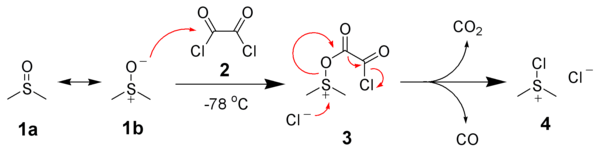
The first step of the Swern oxidation is the low-temperature reaction of dimethyl sulfoxide (DMSO), 1a, formally as resonance contributor 1b, with oxalyl chloride, 2. The first intermediate, 3, quickly decomposes giving off CO2 and CO and producing chloro(dimethyl)sulfonium chloride, 4.
After addition of the alcohol 5, the chloro(dimethyl)sulfonium chloride 4 reacts with the alcohol to give the key alkoxysulfonium ion intermediate, 6. The addition of at least 2 equivalents of base — typically triethylamine — will deprotonate the alkoxysulfonium ion to give the sulfur ylide 7. In a five-membered ring transition state, the sulfur ylide 7decomposes to give dimethyl sulfide and the desired ketone (or aldehyde) 8.
Dimethyl sulfide, a byproduct of the Swern oxidation, is one of the most foul odors known in organic chemistry. Human olfactory glands can detect this compound in concentrations as low as 0.02 to 0.1 parts per million. A simple remedy for this problem is to rinse used glassware with bleach (usually containing sodium hypochlorite), which will oxidize the dimethyl sulfide, eliminating the smell.
The reaction conditions allow oxidation of acid-sensitive compounds, which might decompose under the acidic conditions of a traditional method such as Jones oxidation. For example, in Thompson & Heathcock’s synthesis of the sesquiterpene isovelleral,the final step uses the Swern protocol, avoiding rearrangement of the acid-sensitive cyclopropanemethanol moiety.
Rapid, exothermic reactions are challenging to do in batch reactors. Reagents such as organometallics, strong bases, and highly active electrophiles are often added slowly to a reaction mixture under energy-intensive cryogenic conditions to prevent an uncontrollable exotherm. Quenching of these high-energy reagents may again require low temperature. This issue is scale dependent,1 and without proper precautions, both the likelihood and hazard of a runaway reaction increase with the size of a reactor.
The high surface area to volume ratio found in flow reactors makes heat transfer more efficient than in batch, allowing rapid removal of thermal energy given off. These features serve to give the chemist or engineer more control over reaction temperature and reduces the risk of thermal runaway.
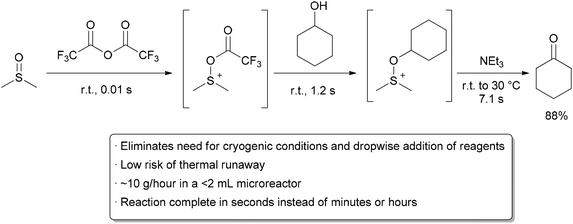
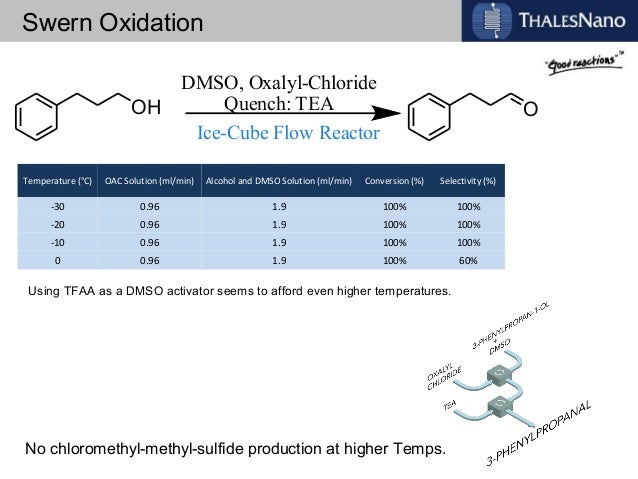
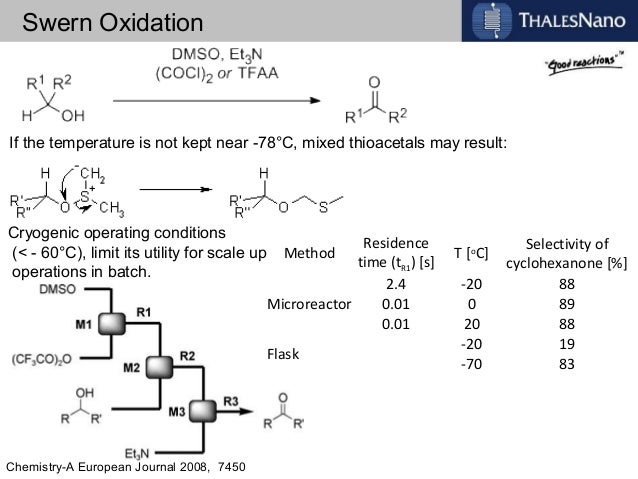
Many instances have been reported of reactions being performed safely at 0 °C or room temperature in flow that would require cryogenic conditions in batch.2,3,4 This has a further benefit on the overall processing time, as the reaction will occur faster at the elevated temperature and inefficient cooling and warming steps are avoided. A remarkable example demonstrating these principles is the room temperature Swern oxidation reaction by Yoshida and co-workers .5
The Swern reaction is a reliable procedure for converting alcohols to ketones and aldehydes using DMSOactivated by an electrophile (typically COCl2 or TFAA) as the oxidant. In batch, the reaction takes place over three exothermic steps, each of which requires dropwise addition of reagents at cryogenic temperatures.6, 7
PROCESS TO FLOW
When converting the process to flow, the Yoshida group found that the Swern oxidation could be done at room temperature with good yields and purity. Moreover, instead of having reaction times on the order of minutes or hours, the whole process was completed in seconds. They attributed the success of their process to the precise temperature control that can be obtained in flow systems, as well as the ability to quickly transfer unstable intermediates to subsequent steps. Using only a series of syringe pumps, stainless steel tubing, and commercial micromixers, they could prepare over 10 grams of material per hour. Being able to perform reactions on species with very short lifetimes is another general advantage of performing reactions in flow.8
 |
||
| Scheme Room temperature Swern oxidation. | ||
……………
MORE……..

http://thalesnano.com/products/IceCube



…………………
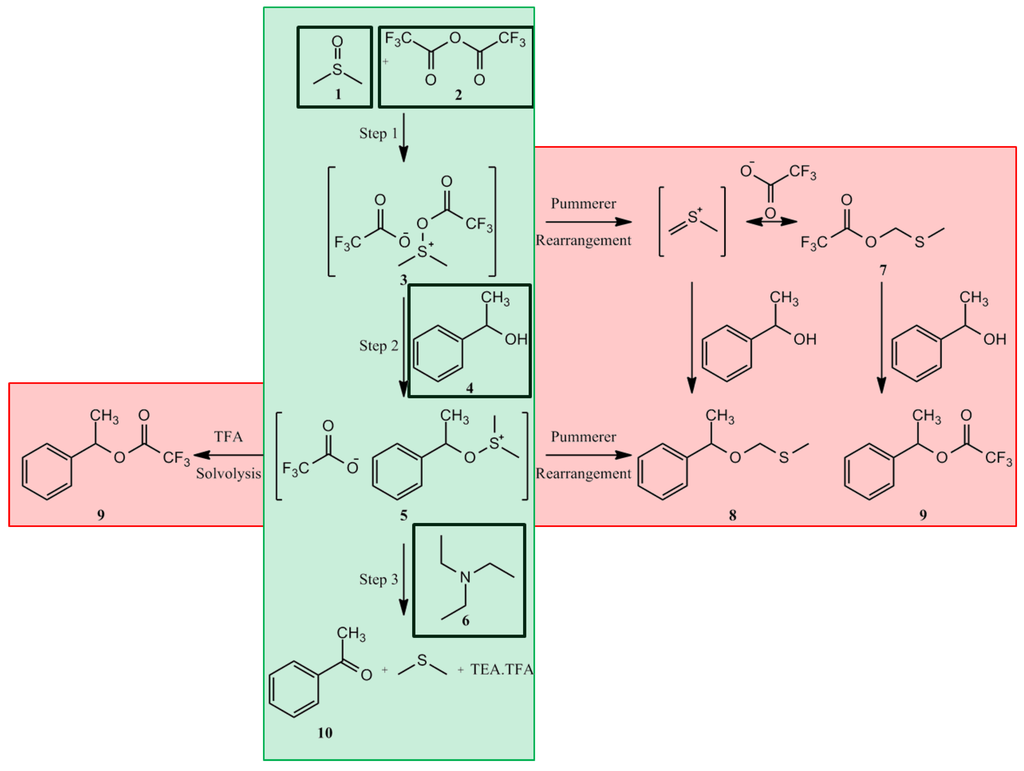
The Swern oxidation. The center column (green background) shows the desired chemical path, with added reagents shown in black boxes. The outer columns (red background) show the potential chemical pathways for side-product formation (8 and 9).
http://www.mdpi.com/2227-9717/2/1/24/htm
REF
- R. L. Hartman, J. P. McMullen and K. F. Jensen, Angew. Chem., Int. Ed., 2011, 50, 7502–7519
- V. Hessel, C. Hofmann, H. Löwe, A. Meudt, S. Scherer, F. Schönfeld and B. Werner, Org. Process Res. Dev., 2004, 8, 511–523 Search PubMed.
- A. Nagaki, Y. Tomida, H. Usutani, H. Kim, N. Takabayashi, T. Nokami, H. Okamoto and J.-i. Yoshida, Chem.–Asian J., 2007, 2, 1513–1523
- T. Gustafsson, H. Sörensen and F. Pontén, Org. Process Res. Dev., 2012, 16, 925–929 Search PubMed.
- T. Kawaguchi, H. Miyata, K. Ataka, K. Mae and J.-I. Yoshida, Angew. Chem., Int. Ed., 2005, 44, 2413–2416
- A. K. Sharma and D. Swern, Tetrahedron Lett., 1974, 15, 1503–1506 Search PubMed.
- A. K. Sharma, T. Ku, A. D. Dawson and D. Swern, J. Org. Chem., 1975, 40, 2758–2764
- J.-i. Yoshida, Chem. Rec., 2010, 10, 332–341
सुकून उतना ही देना प्रभू, जितने से जिंदगी चल जाये। औकात बस इतनी देना, कि औरों का भला हो जाये।
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
Join me on Facebook FACEBOOK
 amcrasto@gmail.com
amcrasto@gmail.com
 LIONEL MY SON
LIONEL MY SON
जिंदगी चल जाये।
औकात बस इतनी देना,
कि औरों का भला हो जाये।















1612-1880/asset/cover.gif?v=1&s=cc68b12e733d21d6415eeec10551f83cb03996eb)