DOI: 10.1039/C8GC00008E, Communication
 Open Access
Open Access
 This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
Utilization of gaseous carbon dioxide as a C1-building block in the biocatalytic ortho-carboxylation of a phenol.
The content of this RSS Feed (c) The Royal Society of Chemistry
Pressurized CO2 as a carboxylating agent for the biocatalytic ortho-carboxylation of resorcinol
Abstract
The utilization of gaseous carbon dioxide instead of bicarbonate would greatly facilitate process development for enzyme catalyzed carboxylations on a large scale. As a proof-of-concept, 1,3-dihydroxybenzene (resorcinol) was carboxylated in the ortho-position using pressurized CO2 (∼30–40 bar) catalyzed by ortho-benzoic acid decarboxylases with up to 68% conversion. Optimization studies revealed tight pH-control and enzyme stability as the most important determinants.
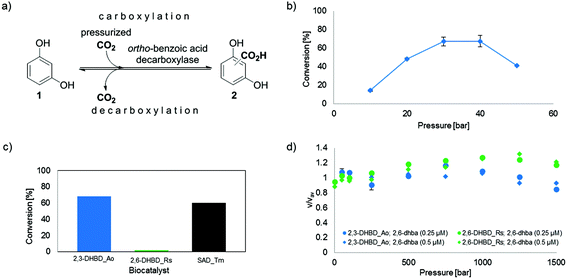 |
||
Fig. 1 (a) Enzyme-catalyzed de/carboxylation of resorcinol (1). Carboxylated product 2 is a mixture of regio-isomeric 2,6- (2,6-dhba, 2a) and 2,4-dihydroxybenzoic acid (2,4-dhba, 2b) with a ratio of 3![[thin space (1/6-em)]](http://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](http://www.rsc.org/images/entities/char_2009.gif) 4;10b (b) CO2 pressure dependence of the carboxylation of 1 using 2,3-DHBD_Ao; (c) carboxylation activity of decarboxylases with CO2 (30 bar) using 1 as a substrate; (d) stopped-flow measurements of the decarboxylation of 2,6-dhba (2a) with pressure-pretreated (≤1.5 kbar) 2,3-DHBD_Ao and 2,6-DHBD_Rs. 4;10b (b) CO2 pressure dependence of the carboxylation of 1 using 2,3-DHBD_Ao; (c) carboxylation activity of decarboxylases with CO2 (30 bar) using 1 as a substrate; (d) stopped-flow measurements of the decarboxylation of 2,6-dhba (2a) with pressure-pretreated (≤1.5 kbar) 2,3-DHBD_Ao and 2,6-DHBD_Rs. |
||
Conclusion
In summary, we have demonstrated that pressurized carbon dioxide can be used directly as a carboxylating agent in the enzyme catalyzed o-carboxylation of a phenol as an alternative to the high concentration of bicarbonate. Two enzyme candidates (2,3-DHBD_Ao and SAD_Tm) readily accepted the alternative CO2 source for the carboxylation of the model substrate resorcinol. In contrast, 2,6-DHBD_Rs was inapplicable under CO2 pressure due to irreversible inactivation, which was correlated to a decrease in pH caused by the dissociation of H2CO3.
Overall, the use of pressurized CO2 gas significantly improves the efficiency of biocatalytic carboxylations and facilitates downstream-processing of this benign and sustainable approach in using CO2 as a carbon feedstock for the synthesis of organic acids.

DOCTORAL STUDENT,
Katharina Plasch
tel: +43-316-380-8646
e-mail:katharina.plasch(at)uni-graz.at

Kurt Faber
Institute of Chemistry – Organic & Bioorganic Chemistry
Karl Franzens Universität Graz
Heinrichstrasse 28
8010 Graz
e-Mail: kurt.faber@uni-graz.at
phone: +43 316 380 5332
fax: +43 316 380 9840
web: http://biocatalysis.uni-graz.at
Kurt Faber, born 1953 in Klagenfurt (Carinthia/Austria), studied chemistry at the University of Graz, where he received his PhD in 1982. After a post-doctoral fellowship from 1982 to 1983 in St. John’s (Canada) he continued his career at the University of Technology in Graz, where he became associate professor in 1997. The following year he was appointed full professor at the University of Graz, where he since heads a research group devoted to the use of biocatalysts for the transformation of non-natural compounds. He was a visiting scientist at University of Tokyo (1987/1988), Exeter University (1990), University of Trondheim (1994), Stockholm University (2001) and University of Minnesota (2005).

Silvia M. Glueck
Silvia M. Glueck, born 1973 in Bruck/Mur (Austria), studied Chemistry at the University of Graz, where she received her PhD under the supervision of Prof. K. Faber in 2004. From 2005 to 2006 she worked as a post-doctoral fellow with Prof. N. J. Turner at the University of Edinburgh and the University of Manchester (MIB). In 2007 she returned to the University of Graz to join Prof. W. Kroutil, and in 2008 she became Research Scientist at the Research Centre Applied Biocatalysis in the group of Prof. K. Faber. Her research interests are focusing on biocatalysis, molecular biology and organic synthesis.

THE ELK CREW
We keep science in motion…
RESEARCHERS
Emmanuel Cigan • Elisabeth Eger • Judith Farnberger • Michael Fuchs • Somayyeh Gandomkar • Silvia Glueck • Christopher Grimm • Barbara Grischek • Mélanie Hall •Valentina Jurkaš • Lucas Hammerer • Barbara Larissegger • Haifeng Liu • Lía Martínez Montero • Stefan Payer • Philipp Petermeier • Mathias Pickl • Katharina Plasch • Jakob Pletz • Hannah Pollak • Silvan Poschenrieder • Tamara Reiter • Luca Schmermund • Joerg Schrittwieser • Erika Tassano • Gábor Tasnádi • Stefan Velikogne • Christoph Winkler •
//////////
Pressurized CO2 as a carboxylating agent for the biocatalytic ortho-carboxylation of resorcinol
K. Plasch, G. Hofer, W. Keller, S. Hay, D. J. Heyes, A. Dennig, S. M. Glueck and K. Faber, Green Chem., 2018, 20, 1754
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence. Material from this article can be used in other publications provided that the correct acknowledgement is given with the reproduced material.
Reproduced material should be attributed as follows:
- For reproduction of material from NJC:
[Original citation] – Published by The Royal Society of Chemistry (RSC) on behalf of the Centre National de la Recherche Scientifique (CNRS) and the RSC. - For reproduction of material from PCCP:
[Original citation] – Published by the PCCP Owner Societies. - For reproduction of material from PPS:
[Original citation] – Published by The Royal Society of Chemistry (RSC) on behalf of the European Society for Photobiology, the European Photochemistry Association, and RSC. - For reproduction of material from all other RSC journals:
[Original citation] – Published by The Royal Society of Chemistry.













Sorry, the comment form is closed at this time.