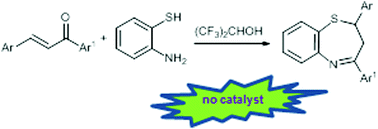
A practical synthesis of 2,3-dihydro-1,5-benzothiazepines
Green Chem., 2017, 19,5703-5707
DOI: 10.1039/C7GC02097J, Paper
Domenico C. M. Albanese, Nicoletta Gaggero, Meng Fei
Hexafluoro-2-propanol as the solvent allows a catalyst free domino approach to 2,3-dihydro-1,5-benzothiazepines in up to 98% yield.
A practical synthesis of 2,3-dihydro-1,5-benzothiazepines
*Corresponding authors
aDepartment of Chemistry, Università degli Studi di Milano, via C. Golgi 19, Milano, Italy
bDepartment of Pharmaceutical Sciences, Università degli Studi di Milano, via L. Mangiagalli 25, Milano, Italy
E-mail:nicoletta.gaggero@unimi.it

Domenico C. M. Albanese
Positionassociate professor
Domenico Albanese received his Ph.D. degree in 1993 with Prof. Dario Landini working on phase transfer catalysis. After short stays at Imperial College London and the Technical University of Denmark, he gained a permanent position at the Università degli Studi di Milano, where he was appointed associate professor in 2008. His research interests include novel developments of phase-transfer catalysis, green chemistry and the development of new environmentally friendly antifouling agents.


 Nicoletta Gaggero Nicoletta Gaggero |
Nicoletta Gaggero received her Ph.D. degree in 1992 working on stereoselective reactions with natural proteins, enzymes and models of enzymes. After working at the Laboratoire de Chimie de Coordination du CNRS of Toulouse, she obtained a permanent position at the Università degli Studi di Milano. Her research interests cover the field of biocatalysis and asymmetric synthesis. |
2,4-Diphenyl-2,3-dihydro-1,5-benzothiazepine (4a)
Yellow solid; mp 114-116 C [lit.1 , 114-115 °C], AcOEt/PE 1:9. 1H NMR (300 MHz, CDCl3,): 3.07 (t, J = 12.6 Hz, 1 H), 3.32 (dd, J = 4.7, 13.1 Hz, 1 H), 4.99 (dd, J = 4.5, 12.0 Hz, 1 H), 7.12-7.17 (m, 1 H), 7.25-7.30 (m, 5 H), 7.44-7.51 (m, 4 H), 7.62 (d, J = 6.1 Hz, 2 H), 8.06 (d, J = 7.5 Hz, 2 H). Isolated Yield: 339 mg, 86%.
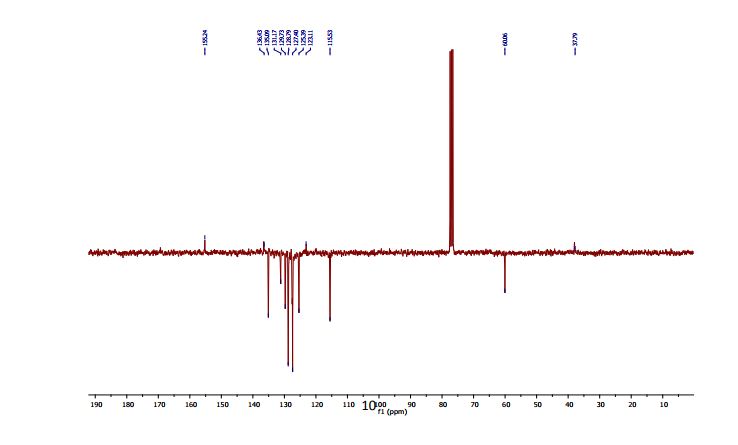
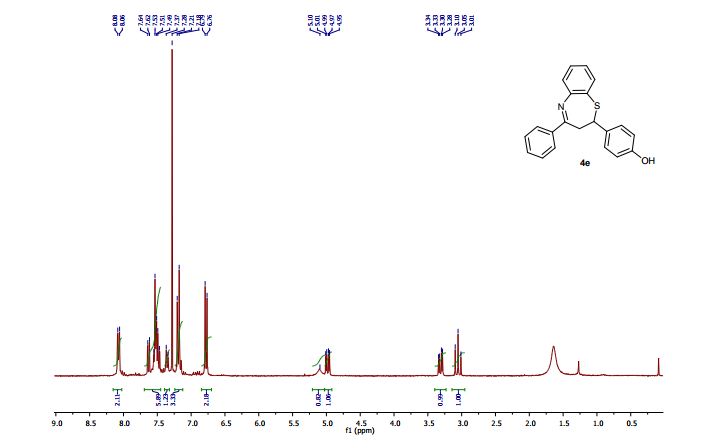
2-(4-Hydroxyphenyl)-4-phenyl-2,3-dihydro-1,5-benzothiazepine (4e)
Light brown solid; mp 131-134 °C. AcOEt/PE 40:60.
1H NMR (CDCl3, 300 MHz): = 3.01 (t, J = 12.7 Hz, 1 H), 3.28 (dd, J = 4.8, 12.9 Hz, 1 H), 4.95 (dd, J = 4.7, 12.5 Hz, 1 H), 5.10 (bs, 1 H), 6.76 (d, J = 8.5 Hz, 2 H), 7.18-7.21 (m, 3 H), 7.35 (d, J = 8.5 Hz, 1 H), 7.46- 7.55 (m, 4 H), 7.63 (dd, J =1.5, 7.7 Hz, 1 H), 8.06 (m, 2 H).
13C NMR (CDCl3, 75 MHz): 37.99 (CH2), 60.07 (CH), 115.53 (CH), 123.08 (C), 127.40 (CH), 128.79 (CH), 131.17 (CH), 136.54 (C), 141.59 (C), 155.24 (C). IR (KBr): 1599, 2921, 3350 cm-1 .
MS (ESI): m/z= 332.24 (MH)+ .
Anal. Calcd. for C21H17NOS: C, 76.10; H, 5.17; N, 4.23, found: C, 76.21; H, 5.15; N, 4.24.
Isolated Yield: 360 mg, 87%.
‘
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
















Sorry, the comment form is closed at this time.