
E/Z
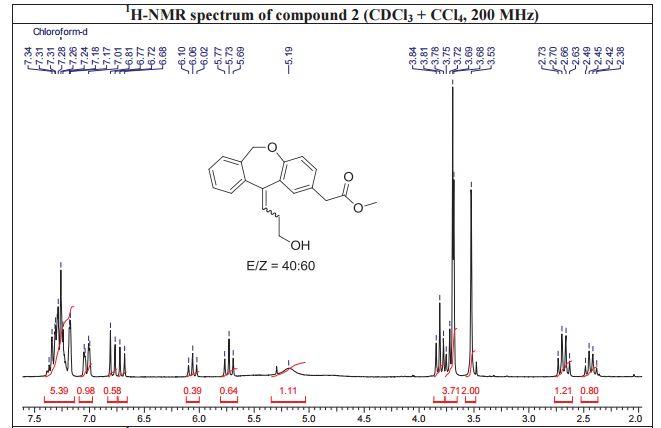
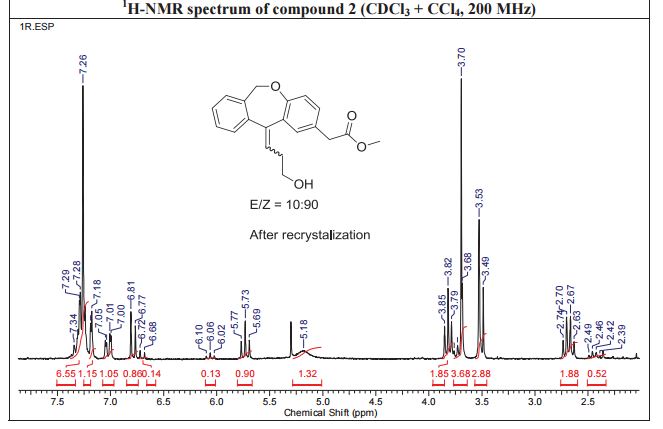
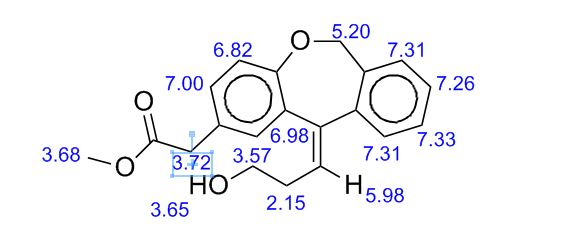
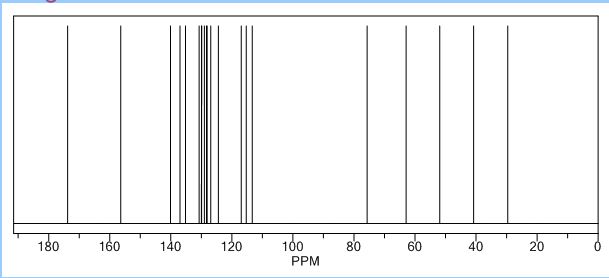
White solid; Ή NMR (200 MHz, CDC13 + CC14): δ 2.38-2.49 ( m,0.8H, E- Form), 2.63-2.73 ( m,1.2H, Z-Form), 3.53 (s, 2H), 3.68 (s, 3H), 3.75 (m, 0.8H, E-Form), 3.81 (t, J=6.3 Hz, 1.2H), 5.19 (brs, 2H), 5.73 (t, J=7.8 Hz, 0.6H, Z-Form), 6.06 (t, J=7.8 Hz, 0.4H, E-Form), 6.70 (d, J=8.2 Hz, 0.4 H, E-Form), 6.79 (d, J=8.2 Hz, 0.6 H, Z- Form), 7.00-7.34 (m, 6H), HRMS m/r. Calculated for C20H2,O4-325.1434, observed- 325.1437.
CLIP 2
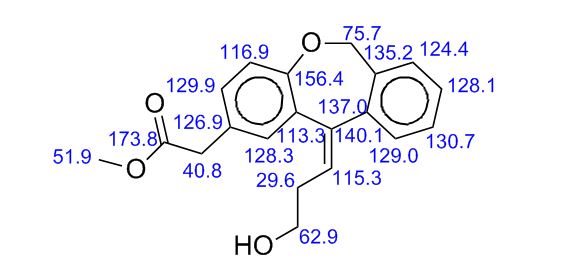
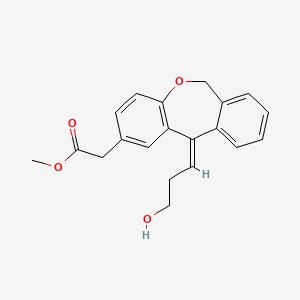
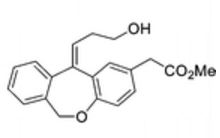
Methyl (Z)-11-[(3-Hydroxy)propylidene]-6,11-dihydrobenz[b,e]oxepin-2-acetate
916243-39-5 cas
- Molecular Weight, 324.37
DOI: 10.1055/s-0033-1340008
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
//////O=C(OC)Cc1ccc2OCc3ccccc3C(=C\CCO)\c2c1













Sorry, the comment form is closed at this time.