Eldecalcitol
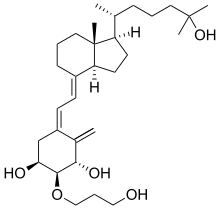
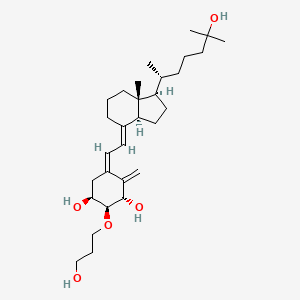
(1S,2S,3S,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(2R)-6-hydroxy-6-methylheptan-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-2-(3-hydroxypropoxy)-4-methylidenecyclohexane-1,3-diol
(1R,2R,3R,5Z,7E)-2-(3-Hydroxypropyloxy)-9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol
| AC1O5QQ2; CAS 104121-92-8; AN-3697; ED 71, Edirol® | |
| Molecular Formula: | C30H50O5 |
|---|---|
| Molecular Weight: | 490.715 g/mol |
APPROVED JAPAN , 2011-01-21, Chugai (Originator) , Roche,Taisho Toyama
Eldecalcitol was approved by Pharmaceuticals and Medicals Devices Agency of Japan (PMDA) on January 21, 2011. It was developed by Chugai Pharmaceutical (a member of Roche) and marketed as Edirol® by Chugai Pharmaceutical and Taisho.
Eldecalcitol is an orally active vitamin D analogue leading to greater absorption of bind calcium. It is usually used to treat osteoporosis.
Edirol® is available as capsule for oral use, containing 0.5 μg or 0.75 μg of free Eldecalcitol, and the recommended dose is 0.75 μg once daily.
ED-71, a vitamin D analog, is a more potent inhibitor of bone resorption than alfacalcidol in an estrogen-deficient rat model of osteoporosis. ED-71, effectively and safely increased lumbar and hip bone mineral density (BMD) in osteoporotic patients who also received vitamin D3 supplementation.
Eldecalcitol is a drug used in Japan for the treatment of osteoporosis.[1] It is an analog of vitamin D.[2] Osteoporosis is a common bone disease among the older generation, with an estimated prevalence of over 200 million people.[1] This condition often results in bone fractures due to abnormally low bone mass density, and is a leading cause of disability, especially among developed countries with longer average life spans. Osteoporosis is more common in women than with men.
Discovery
Chugai Pharmaceutical/Roche are the originators of the medicinal drug eldecalcitol through Taisho Pharmaceutical Holdings and Chugai Pharmaceutical. The trade name of eldecalcitol is Edirol, and its Chemical Abstracts Service (CAS) registry number is 104121-92-8. Eldecalcitol was approved for use in Japan on January 2011. The approval came from the Japanese Ministry of Health, Labor, and Welfare for the objective of a treatment for osteoporosis.[3]
Effects
Clinical trials have suggested that eldecalcitol, a vitamin D analog, has strong effects to reduce calcium reabsorption into the body from bones, therefore increasing bone mineral density, and to increase calcium absorption in intestines.[4] In animals, eldecalcitol inhibits the activity of osteoclasts for the function to reduce bone degradation for calcium, while still able to maintain osteoblast function so as to not hinder bone formation.[5] Unlike other vitamin D analogs, eldecalcitol does not significantly suppress parathyroid hormone levels, promising a better treatment for osteoporosis in comparison to other medications.[6] Bone mineral density increases with eldecalcitol use, in addition to strengthening bone structure. This occurs due to the function of the eldecalcitol drug, which decreases bone reabsorption as observed through a bone reabsorption marker. Bone geometry assessments show that eldecalcitol increases cortical bone area in patients with osteoporosis more so than other vitamin D analogs, such as alfacalcidol. There was also the maintenance of thickness of cortical bone mass, strongly indicating that eldecalcitol improves the strength and mass of bone, specifically cortical bone structure.[7] Adverse effects of eldecalcitol include an increase in blood and urinary calcium levels. Abnormally high levels of calcium can lead to problems associated with hypercalcemia.
Treatment for Osteoporosis
Eldecalcitol can be used for the treatment of hypocalcaemia or osteoporosis. Calcium absorption increases with the presence of eldecalcitol by the body, occurring in the intestines, which is useful for those who have low calcium levels. Eldecalcitol is more often used due to its effects to treat osteoporosis. In the aging population, the bone matrix becomes weakened through untreated osteoporosis. This leads to an increased risk of severe fractures that include spinal and hip fractures in addition to vertebral and wrist fractures. This creates a burden on the health care system due to a decline in the quality of life for the individuals that suffer from this condition. Some risk factors leading to the predisposition of developing osteoporosis are previous incidents of bone fractures and a reduction in bone mineral density.[1] These factors expectantly increase as age increases. Bone health is reliant on maintaining physiologically needed levels of calcium, where the body constantly maintains this calcium homeostasis through osteoblast and osteoclast activity. Osteoblast activity serves this function of maintaining appropriate calcium levels by depositing calcium in bones when blood calcium levels are above normal. In contrast, osteoclasts break down bone tissue to increase blood calcium levels if they are low.[8] This activity is performed after absorption of calcium by the body, which requires the actions of vitamin D. The active metabolite of vitamin D, calcitriol, performs its function through interactions with the calcitriol receptor. This nuclear hormone receptor is responsible for calcium absorption which, in turn, is involving in bone depletion and formation. The new analogs of vitamin D, such as eldecalcitol, are observed to have stronger effects in preventing bone loss, fractures, and falls in comparison to calcitriol.[9] Eldecalcitol is even more effective than its counterpart alfacalcidol, another vitamin D analog. Studies have shown eldecalcitol is more effective than alfacalcidol in preventing vertebral and wrist fractures, and even falls, with osteoporotic patients with vitamin D insufficiencies.[10] Eldecalcitol is also more effective at preventing fractures than vitamin D and calcium supplements.[1] Eldecalcitol increases calcium absorption for vitamin D deficient patients, and therefore could be used for osteoporosis treatment for all age groups.
Pharmacology
Analogs of vitamin D are being explored intensely for their regulatory effects on calcium metabolism with the purpose of treating osteoporosis, a skeletal disease associated with low bone mass and deterioration of bone tissue. Vitamin D is imperative for absorption of calcium to maintain bone strength.
Mechanism of Action
Eldecalcitol is an orally administered drug to patients, which binds to vitamin D receptors and binding protein for the goal of achieving greater specificity to bind calcium for its absorption. This greater affinity is 2.7-fold that of the active vitamin D form of calcitriol. Eldecalcitol is readily absorbed into the body, with a long elimination half-life of over eight hours, reaching maximum absorption in 3.4 hours.[1]
Dosage
Eldecalcitol is present in the form of pills for oral administration. In preclinical models with healthy male volunteers, oral doses of eldecalcitol ranged from 0.1 to 1.0 micrograms once daily to show an increase in bone mineral density.[11] Preclinical trials show improvements for doses at 0.5 and 0.75 micrograms, which are the recommended dosage amounts for the Edirol product as approved by the Japanese Ministry of Health, Labor, and Welfare for treating osteoporosis.[3]
Chemistry
The class of eldecalcitol is a vitamin D3 derivative. This molecule has a molecular weight of 490.71 grams per mole. The eldecalcitol analog of calcitriol, contains a hydroxypropyl group in the lower cyclohexane ring. The synthesis of eldecalcitol incorporates two units assembled together. The IUPAC names include (3S, 4S, 5R)-oct-1-en-7-yne-3,4,5-triol that is fused to a bicyclic system, (R)-6-((1R, 3aR, 7aR, E)-4-(bromomethylene)-7a-methyloctahydro-1H-inden-1-yl)-2-methylheptan-2-ol. The assembly process includes a Diels-Alder reaction to give the fully protected eldecalcitol. In order to get the parent molecule, the hydroxyl groups have to be deprotected. The chemistry of eldecalcitol allows for its binding 2.7-fold more potently than calcitriol. In addition, some vitamin D derivatives have been known to inhibit the serum parathyroid hormone. Eldecalcitol only weakly inhibits the serum parathyroid hormone, making it an even more appealing medicinal drug for its physiological uses in the treatment of osteoporosis.[3] Animal studies of eldecalcitol, in ovariectomized rats, show improvements in bone mass while lowering bone reabsorption to demonstrate its effectiveness in osteoporosis treatment.[5]
PAPER
Heterocycles, Vol 92, No. 6, 2016, pp.1013-1029
Published online, 22nd March, 2016
■ Diverse and Important Contributions by Medicinal Chemists to the Development of Pharmaceuticals: An Example of Active Vitamin D3 Analog, Eldecalcitol
Noboru Kubodera*
*International Institute of Active Vitamin D Analogs, 35-6, Sankeidai, Mishima, Shizuoka 411-0017, Japan
Abstract
Presented herein are diverse and important contributions by medicinal chemists to different stages of pharmaceutical development. The conceptual elements reviewed, which are intended for young chemists who engage in drug discovery research, draw upon the author’s experience in developing eldecalcitol, an active vitamin D3 analog used to treat osteoporosis. The review covers exploratory research for a lead candidate compound; process development for practical manufacturing; and synthesis of other compounds relevant to the program, such as tritiated compounds, postulated metabolites, and miscellaneous analogs for mode of action studies.
PAPER
Eldecalcitol [1α,25-dihydroxy-2β-(3-hydroxypropoxy)vitamin D3], an analog of calcitriol (1α,25-dihydroxyvitamin D3), possesses a hydroxypropoxy substituent at the 2β-position of calcitriol. Eldecalcitol has potent biological effects on bone disease such as osteoporosis. The marketing of eldecalcitol has very recently started in Japan. In consideration of this, we have been investigating practical synthesis of eldecalcitol for industrial-scale production. Eldecalcitol was initially synthesized in a linear manner. The 27-step linear sequence was, however, suboptimal due to its lengthiness and low overall yield (ca. 0.03%). Next, we developed a convergent approach based on the Trost coupling reaction, in which the A-ring fragment (ene-yne part obtained in 10.4% overall yield) and the C/D-ring fragment (bromomethylene part obtained in 27.1% overall yield) are coupled to produce the triene system of eldecalcitol (15.6%). Although the overall yield of the convergent synthesis was better than that of the linear synthesis, significant improvements were still necessary. Therefore, additional biomimetic studies were investigated. Process development for the practical production of eldecalcitol is described herein.
http://ar.iiarjournals.org/content/32/1/303/F3.expansion.html
Convergent synthesis of eldecalcitol (5) by coupling A-ring fragment 37 with C/D-ring fragment 40. Reagents and conditions: a: HO(CH2)3OH/t-BuOK, 120°C. b: t-BuCOCl/pyridine/CH2Cl2, rt. c: H2/Pd(OH)2/MeOH, rt. d: Me2C(OMe)2/TsOH/acetone, rt. e: DMSO/(COCl)2/CH2Cl2, −60°C. f: CH2=CHMgBr/THF, −60°C. g: t-BuCOCl/Et3N/DMAP/CH2Cl2, rt. h: 1 M HCl/MeOH, rt. i: Ph3P/DEAD/benzene, reflux. j: LiC ≡ CTMS/BF3-OEt2, −78°C. k: 10 N NaOH/MeOH, rt. l: TBSOTf/Et3N/CH2Cl2, 0°C. m: TESOTf/Et3N/CH2Cl2, 0°C. n: O3/CH2Cl2/MeOH, −78°C then NaBH4/MeOH, −78°C. o: NMO/TPAP/4Ams/CH2Cl2, rt. p: Ph3P+CH2BrBr−/NaHMDS/ THF, −60°C to rt. q: (dba)3Pd2-CHCl3/PPh3/Et3N/toluene, reflux. r: TBAF/THF/toluene, reflux.

Industrial synthesis of alfacalcidol (4) and biomimetic synthesis of eldecalcitol (5) from cholesterol (42). Reagents and conditions: a: [Al(Oi-Pr)3]/cyclohexanone. b: DDQ/AcOEt. c: NaOEt/EtOH. d: NaBH4/MeOH/THF. e: Ac2O/DMPA/pyridine, rt. f: NBS/AIBN/n-hexane, reflux. g: γ-collidine/toluene, reflux. h: KOH/MeOH, rt. i: PTAD/CH2Cl2, rt. j: TBSCl/imidazole. k: MCPBA/CH2Cl2. l: DMI, 140°C. m: TBAF/THF. n: NaBH4/EtOH. o: 400 W high pressure mercury lamp/THF, 0°C then reflux without mercury lamp. p: HO(CH2)3OH/t-BuOK, 110°C. q: Microbial 25-hydroxylation.
ROUTE1
1. US4666634A / EP184206A2.
1. Anticancer. Res. 2012, 32, 303-310.
2. Drugs. Fut. 2005, 30, 450-461.
1. Bioorg. Med. Chem. Lett. 1997, 7, 2871-2874.
2. Anticance. Res. 2009, 29, 3571-3578.
References
- Sanford, M; McCormack, PL (2011). “Eldecalcitol: A review of its use in the treatment of osteoporosis”. Drugs 71 (13): 1755–70. doi:10.2165/11206790-000000000-00000. PMID 21902297.
- Hatakeyama, S; Yoshino, M (2010). “Synthesis and preliminary biological evaluation of 20-epieldecalcitol [20-epi-1α,25-dihydroxy-2β-(3-hydroxypropoxy)vitamin D3: 20-epi-ED-71]”. The Journal of Steroid Biochemistry and Molecular Biology 121 (1–2): 25–28.doi:10.1016/j.jsbmb.2010.03.041. PMID 20304058.
- Robichaud; Stamford; Weinstein; McAlpine; Primeau; Lowe; Bernstein; Bronson; Manoj, Desai (2012). Annual Reports in Medicinal Chemistry 47 (1st ed.). San Diego: Elsevier Inc. pp. 529–531. ISBN 9780123964922.
- Nogachi, Y; Kawate, H; Nomura, M; Takayanagi, R (2013). “Eldecalcitol for the treatment of osteoporosis”. Europe PubMed Central 8: 1313–1321. doi:10.2147/CIA.S49825.
- Smith, S; Doyle, N; Boyer, M; Chouinard, L; Saito, H (2013). “Eldecalcitol, a vitamin D analog, reduces bone turnover and increases trabecular an cortical bone mass, density, and strength in ovariectomized cynomolgus monkeys”. Bone 57 (1): 116–122.doi:10.1016/j.bone.2013.06.005. PMID 23774444.
- Harada, S; Uno, S; Takahashi, F; Saito, H (2010). “Eldecalcitol is less effective in suppressing parathyroid hormone compared to calcitriol in vivo“. The Journal of Steroid Biochemistry and Molecular Biology 121 (1–2): 281–283.doi:10.1016/j.jsbmb.2010.04.001. PMID 20398764.
- Nakamura, T; Takano, T; Fukunaga, M; Shiraki, M; Matsumoto, T (2013). “Eldecalcitol is more effective for the prevention of osteoporotic fractures than alfacalcidol”. Journal of Bone and Mineral Metabolism 31 (4): 417–422. doi:10.1007/s00774-012-0418-5.PMC 3709079. PMID 23575909.
- Matsuo, K; Irie, N (2008). “Osteoclast-osteoblast communication”. Archives of Biochemistry and Biophysics 473 (2): 201–209. doi:10.1016/j.abb.2008.03.027.PMID 18406338.
- Saito, H; Takeda, S; Amizuka, N (2013). “Eldecalcitol and calcitriol stimulates ‘bone minimodeling,’ focal bone formation without prior bone resorption, in rat trabecular bone”.The Journal of Steroid Biochemistry and Molecular Biology 136 (1): 178–182.doi:10.1016/j.jsbmb.2012.10.004.
- Matsumoto, T; Ito, M; Hayashi, Y; Hirota, T; Tanigawara, Y; Sone, T; Fukunaga, M; Shiraki, M; Nakamura, T (2011). “A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures—A randomized, active comparator, double-blind study”. Bone49 (4): 605–612. doi:10.1016/j.bone.2011.07.011. PMID 21784190.
- Harada, S; Mizoguchi, T; Kobayashi, Y; Nakamichi, Y; Takeda, S; Sakai, S; Takahashi, F; Saito, H; Yasuda, H; Udagawa, N; Suda, T; Takahashi, N (2012). “Daily administration of eldecalcitol (ED-71), an active vitamin D analog, increases bone mineral density by suppressing RANKL expression in mouse trabecular bone”. Journal of Bone and Mineral Research 27 (1): 461–473. doi:10.1002/jbmr.555.
| No. | Major Technical Classification | Publication No. | Patent No. | Legal Status | Filling Date | Estimated Expiry Date |
| 1 | Preparation | CN85108857A | CN1008368B | Granted/expired | 1985/12/4 | 2005/12/4 |
| 2 | Crystal | CN1223639A | CN1216861C | Granted | 1997/6/16 | 2017/6/16 |
| 3 | Preparation | CN1637017A | CN1276927C |
| Patent ID | Date | Patent Title |
|---|---|---|
| US7927613 | 2011-04-19 | Pharmaceutical co-crystal compositions |
| US7323580 | 2008-01-29 | CRYSTALS OF A VITAMIN D DERIVATIVE AND A METHOD FOR THE PREPARATION THEREOF |
| US7235679 | 2007-06-26 | Crystals of a vitamin D derivative and a method for the preparation thereof |
| EP0924199 | 2006-05-10 | CRYSTALS OF VITAMIN D DERIVATIVES AND PROCESS FOR THE PREPARATION THEREOF |
| US2005009794 | 2005-01-13 | Crystals of a vitamin D derivative and a method for the preparation thereof |
| US6831183 | 2004-12-14 | Crystals of a vitamin D derivative and a method for the preparation thereof |
| US6448421 | 2002-09-10 | CRYSTALS OF VITAMIN D DERIVATIVES AND PROCESS FOR THE PREPARATION THEREOF |
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
(1S,2S,3S,5Z,7E)-2-(3-Hydroxypropoxy)-9,10-secocholesta-5,7,10-triene-1,3,25-triol
|
|
| Clinical data | |
| Trade names | Edirol |
| Identifiers | |
| CAS Number | 104121-92-8 |
| ATC code | None |
| PubChem | CID 6438982 |
| ChemSpider | 4943418 |
| Chemical data | |
| Formula | C30H50O5 |
| Molar mass | 490.715 g/mol |
///////////eldecalcitol, active vitamin D3 analog, treat osteoporosis, AC1O5QQ2, 104121-92-8, AN-3697, ED 71, ED-71, Edirol®, PMDA, JAPAN
O[C@H]1CC(\C(=C)[C@H](O)[C@H]1OCCCO)=C\C=C2/CCC[C@]3([C@H]2CC[C@@H]3[C@H](C)CCCC(O)(C)C)C
OR
CC(CCCC(C)(C)O)C1CCC2C1(CCCC2=CC=C3CC(C(C(C3=C)O)OCCCO)O)C


















.jpg)