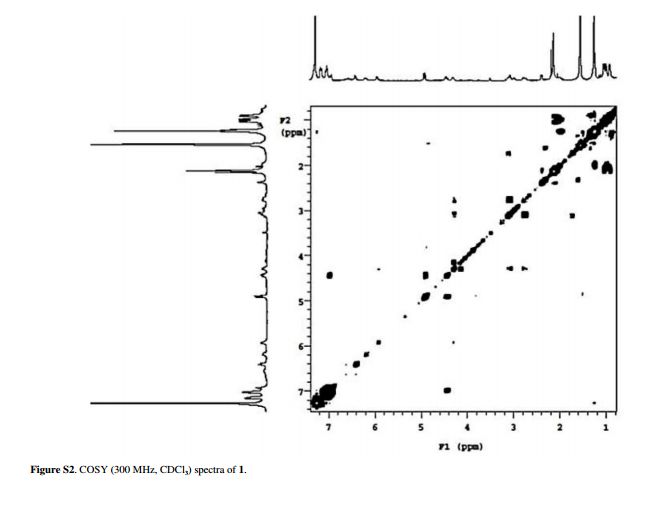
Figure 2 Selected key HMBC (1H 13C) and NOESY (1H 1H) correlations for ixorine (1).
Figure 1 Structures of compounds 1–4 isolated from the branches of I. brevifolia.
Ixorine (1)
HRESIMS m/z, calcd.: C30H40N4O4 [M + H]+: 521.3044; found: 521.3049; [α]D20 = -292.3 (c 0.001, CHCl3); 1H NMR (300 MHz, CDCl3) and 13C NMR (75 MHz, CDCl3), see Table 1.
Table 1 NMR spectroscopic data (300 MHz, CDCl3) for ixorine (1)
| Position | δC | δH (mult., J in Hz) | HMBC | COSY |
|---|---|---|---|---|
| 1 | 156.3 | − | H-14, H-16 | − |
| 2 | − | − | − | − |
| 3 | 81.7 | 4.91 (dd, 8.0, 2.1) | H-4, H-18, H-19 | H-4 |
| 4 | 55.6 | 4.44 (dd, 9.0, 8.0) | − | H-20 (NH) |
| 5 | 171.6 | − | H-6 (NH) | − |
| 6 | − | 5.94 (d, 6.3, NH) | − | − |
| 7 | 55.2 | 4.30 (m) | H-28 | H-28 |
| 8 | 167.2 | − | H-28 | − |
| 9 | − | 6.20 (sl, NH) | − | − |
| 10 | 125.7 | 6.56 (m) | − | − |
| 11 | 118.4 | 6.40 (d, 6.6) | H-13 | − |
| 12 | 131.8 | − | H-14 | − |
| 13 | 131.7 | 7.03 (m) | − | H-14 |
| 14 | 121.9 | 7.15 (m) | − | − |
| 15 | 122.7 | 7.03 (m) | − | H-16 |
| 16 | 130.2 | 6.92 (m) | − | − |
| 17 | 29.3 | 2.00 (m) | H-18, H-19 | H-18, H-19 |
| 18 | 20.4 | 1.25 (d, 7.2) | H-17, H-19 | − |
| 19 | 15.2 | 1.00 (d, 6.6) | H-17 | − |
| 20 | − | 6.94 (m, NH) | − | − |
| 21 | 172.4 | − | H-22, H-23 | − |
| 22 | 75.2 | 2.40 (d, 4.2) | H-24, H-25, H-26, H-27 | H-26, H-27 |
| 23 | 27.8 | 2.05 (m) | H-24, H-25 | H-24, H-25 |
| 24 | 21.0 | 1.05 (d, 6.9) | − | − |
| 25 | 17.6 | 0.93 (d, 6.6) | − | − |
| 26 | 43.1 | 2.14 (s) | H-27 | − |
| 27 | 43.1 | 2.14 (s) | − | − |
| 28 | 37.1 | 2.76 (dd, 13.8, 4.5)/3.07 (m) | H-30, H-30’ | H-28 |
| 29 | 135.7 | − | H-7, H-28, H-31, H-31’ | − |
| 30, 30’ | 129.6 | 7.15 (m) | − | H-31, H-31’ |
| 31, 31’ | 129.0 | 7.34 (m) | − | − |
| 32 | 127.4 | 7.24 (m) | − | H-30, H-30’ |
Journal of the Brazilian Chemical Society
On-line version ISSN 1678-4790
J. Braz. Chem. Soc. vol.27 no.4 São Paulo Apr. 2016
http://dx.doi.org/10.5935/0103-5053.20150326
ARTICLES
Ixorine, a New Cyclopeptide Alkaloid from the Branches of Ixora brevifolia
aDepartamento de Química, Universidade Estadual de Maringá, Av. Colombo 5790, 87020-900 Maringá-PR, Brazil
bInstituto de Química, Universidade Federal de Goiás, Campus II, Samambaia, 74001-970 Goiânia-GO, Brazil
cDepartamento de Análises Clínicas e Biomedicina, Universidade Estadual de Maringá, Av. Colombo 5790, 87020-900 Maringá-PR, Brazil
ABSTRACT
The isolation and structure determination of new cyclic peptide alkaloid ixorine, along with five known constituents frangulanine, syringaresinol, cinnamtannin B-1, daucosterol and mannitol from the branches of Ixora brevifolia are described. The cyclic peptide frangulanine is being described for the first time in the Rubiaceae family. The structures were elucidated on their spectral data basis, mainly one- (1H, 13C, DEPT) and two-dimensional (COSY, NOESY, HSQC and HMBC) nuclear magnetic resonance (NMR) and by comparison with data from the literature. The mixture of two cyclopeptide alkaloids showed weak activity against Leishmania amazonensis……..http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-50532016000400753&lng=en&nrm=iso&tlng=en
Key words: Ixora brevifolia, Rubiaceae, cyclopeptide alkaloids, Leishmania
see……….http://www.scielo.br/pdf/jbchs/v27n4/0103-5053-jbchs-27-04-0753-suppl01.pdf
SUPPLEMENTARY INFORMATION
1D and 2D NMR spectra for compounds 1–2 are available free of charge online
0103-5053-jbchs-27-04-0753-suppl01.pdf
*e-mail: smoliveira@uem.br
////