Fispemifene, HM 101
Fispemifene; UNII-3VZ2833V08;
cas 341524-89-8
| Molecular Formula: | C26H27ClO3 |
|---|---|
| Molecular Weight: | 422.94378 g/mol |
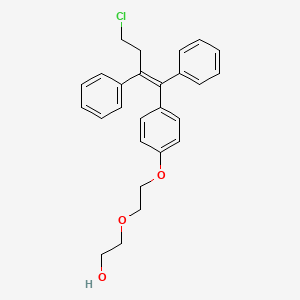
2-[2-[4-[(Z)-4-chloro-1,2-diphenylbut-1-enyl]phenoxy]ethoxy]ethanol
Treatment of Hypogonadism
Androgen Decline in the Aging Male (Andropause) in phase 2
Fispemifene is the Z-isomer of the compound of formula (I)
WO 01/36360 describes a group of SERMs, which are tissue-specific estrogens and which can be used in women in the treatment of climacteric symptoms, osteoporosis, Alzheimer’s disease and/or cardiovascular diseases without the carcinogenic risk. Certain compounds can be given to men to protect them against osteoporosis, cardiovascular diseases and Alzheimer’s disease without estrogenic adverse events (gynecomastia, decreased libido etc.). Of the compounds described in said patent publication, the compound (Z)-2-{2-[4-(4-chloro-1,2-diphenylbut-1-enyl)phenoxy]ethoxy}ethanol (also known under the generic name fispemifene) has shown a very interesting hormonal profile suggesting that it will be especially valuable for treating disorders in men. WO 2004/108645 and WO 2006/024689 suggest the use of fispemifene for treatment or prevention of age-related symptoms in men, such as lower urinary tract symptoms and diseases or disorders related to androgen deficiency in men.
Quatrx had been conducting phase II clinical development for the treatment of androgen decline in the aging male. Unlike testosterone replacement therapies that are typically topical or injection therapies, fispemifene is an oral treatment and is not a formulation of testosterone. Fispemifene utilizes the body’s normal feedback mechanism to increase testosterone levels. Originally developed at Hormos, QuatRx gained rights to the drug candidate following a merger of the companies pursuant to which Hormos became a wholly-owned subsidiary of QuatRx.
Known methods for the syntheses of compounds like ospemifene and fispemifene include rather many steps. WO 02/090305 describes a method for the preparation of fispemifene, where, in a first step, a triphenylbutane compound with a dihydroxysubstituted butane chain is obtained. This compound is in a second step converted to a triphenylbutene where the chain is 4-chlorosubstituted. Then the desired Z-isomer is crystallized. Finally, the protecting group is removed to release the ethanol-ethoxy chain of the molecule.
Fispemifene is a selective estrogen receptor modulator (SERM) studied in phase II clinical trials at Forendo Pharma for the treatment low testosterone in men. The compound is also in phase II clinical studies at Apricus for the treatment of men with secondary hypogonadism.
In 2013, Forendo Pharma acquired the drug from Hormos Medical for the treatment of male low testosterone.
In 2014, Apricus Biosciences acquired U.S. rights for development and commercialization
PATENT
https://www.google.com/patents/US7504530
EXAMPLE 2 2-{2-[4-(4-Chloro-1,2-diphenyl-but-1-enyl)-phenoxy]-ethoxy}-ethanol (Compound I)
{2-[4-(4-Chloro-1,2-diphenyl-but-1-enyl)-phenoxy]-ethoxy}-acetic acid ethyl ester was dissolved in tetrahydrofuran at room temperature under nitrogen atmosphere. Lithium aluminium hydride was added to the solution in small portions until the reduction reaction was complete. The reaction was quenched with saturated aqueous ammonium chloride solution. The product was extracted into toluene, which was dried and evaporated in vacuo. The residue was purified with flash chromatography with toluene/triethyl amine (9.5:0.5) as eluent. Yield 68%.
1H NMR (200 MHz, CDCl3):
2.92 (t, 2H, ═CH 2CH2Cl),
3.42 (t, 2H, ═CH2 CH2 Cl),
3.59-3.64 (m, 2H, OCH2CH2O CH2CH 2OH),
3.69-3.80 (m, 4H, OCH2 CH 2OCH 2 CH2OH),
3.97-4.02 (m, 2H, OCH2CH2OCH2CH2OH),
6.57 (d, 2H, aromatic proton ortho to oxygen),
6.78 (d, 2H, aromatic proton meta to oxygen),
7.1-7.43 (m, 10H, aromatic protons).
………….
PATENT
WO 2001036360
https://www.google.com/patents/WO2001036360A1?cl=en
……………
PATENT
WO 2002090305
http://www.google.co.in/patents/WO2002090305A1?cl=en
EXAMPLE
a) [2-(2-chloroethoxy)ethoxymethyl]benzene
is prepared from benzyl bromide and 2-(2-chloroethoxy)ethanol by the method described in literature (Bessodes, 1996).
b) {4-[2-(2-Benzyloxyethoxy)ethoxy]phenyl}phenylmethanone

The mixture of 4-hydroxybenzophenone (16.7 g, 84.7 mmol) and 48 % aqueous sodium hydroxide solution (170 ml) is heated to 80 °C. Tetrabutylammonium bromide (TBABr) (1.6 g, 5.1 mmol) is added and the mixture is heated to 90 °C. [2-(2-Chloroethoxy)ethoxymethyl]benzene (18. g, 84.7 mmol) is added to the mixture during 15 min and the stirring is continued for additional 3.5 h at 115-120 °C. Then the mixture is cooled to 70 °C and 170 ml of water and 170 ml of toluene are added to the reaction mixture and stirring is continued for 5 min. The layers are separated and the aqueous phase is extracted twice with 50 ml of toluene. The organic phases are combined and washed with water, dried with sodium sulphate and evaporated to dryness. Yield 31.2 g.
Another method to prepare {4-[2-(2-benzyloxyethoxy)ethoxy]phenyl}phenyl- methanone is the reaction of 2-(2-benzyloxyethoxy)ethyl mesylate with 4- hydroxybenzophenone in PTC-conditions.
Η NMR (CDCI3): 3.64-3.69 (m, 2H), 3.74-3.79 (m, 2H), 3.90 (dist.t, 2H), 4.22 (dist.t, 2H), 4.58 (s, 2H), 6.98 (d, 2H), 7.28-7.62 (m, 8H), 7.75 (td, 2H), 7.81 (d, 2H).
c) 1- {4-[2-(2-Benzyloxyethoxy)ethoxy]phenyl} – 1 ,2-diphenyl -butane- 1 ,4-diol
 R = BENZYL
R = BENZYL
Lithium aluminum hydride (1.08 g, 28.6 mmol) is added into dry tetrahydrofuran (60 ml) under nitrogen atmosphere. Cinnamaldehyde (6.65 g, 50 mmol) in dry tetrahydrofuran (16 ml) is added at 24-28 °C. The reaction mixture is stirred at ambient temperature for 1 h. {4-[2-(2- Benzyloxyethoxy)ethoxy]phenyl}-phenyl-methanone (14.0 g, 37 mmol) in dry tetrahydrofuran (16 ml) is added at 50-55 °C. The reaction mixture is stirred at 60 °C for 3 h. Most of tetrahydrofuran is evaporated. Toluene (70 ml) and 2 M aqueous hydrogen chloride (50 ml) are added. The mixture is stirred for 5 min and the aqueous layer is separated and extracted with toluene (30 ml). The toluene layers are combined and washed with 2M HC1 and water, dried and evaporated. The product is crystallized from isopropanol as a mixture of stereoisomers (8.8 g, 50 %).
Η NMR (CDCI3 ): 1.75-2.10 (m, 2H), 3.20-4.16 (m, 1 OH), 4.52 and 4.55 (2s, together 2H), 6.61 and 6.88 (2d, together 2H), 6.95-7.39 (m, 15H), 7.49 and 7.57 (2d, together 2H).
d) Z- 1 – {4-[2-(2-Benzyloxyethoxy)ethoxy]phenyl} -4-chloro- 1 ,2-diphenyl-but- 1-ene
 R = BENZYL
R = BENZYL
1 – {4- [2-(2-Benzyloxy-ethoxy)ethoxy]phenyl} – 1 ,2-diphenyl -butane- 1 ,4-diol (10.0 g, 19.5 mmol) is dissolved in toluene (50 ml). Triethylamine (2.17 g, 21.4 mmol) is added to the solution and the mixture is cooled to -10 °C. Thionyl chloride (6.9 g, 58.5 mmol) is added to the mixture at -10 – ±0 °C. The mixture is stirred for 1 hour at 0-5 °C, warmed up to 70 °C and stirred at this temperature for 4 hours. Solvent is evaporated, the residue is dissolved to toluene, washed three times with 1M HC1 solution and twice with water. The Z-isomer of the product is crystallized from isopropanol-ethyl acetate. Yield 3.0 g. The filtrate is purified by flash chromatography to give E-isomer.
Z-isomer: Η NMR (CDCI3): 2.91 (t, 2H), 3.41 (t, 2H), 3.55-3.85 (m, 6H), 3.99 (dist.t, 2H), 4.54 (s, 2H), 6.40 (s, 1H), 6.56 (d, 2H), 6.77 (d, 2H), 7.10- 7.50 (m, 15H)
E-isomer: 1H NMR (CDCI3): 2.97 (t, 2H), 3.43 (t, 2H), 3.65-3.82 (m, 4H), 3.88 (dist.t, 2H), 4.15 (dist.t, 2H), 4.58 (s, 2H), 6.86 -7.45 (m, 19H)
FINAL STEP
e) 2- {2-[4-(4-Chloro- 1 ,2-diphenyl-but- 1 -enyl)phenoxy]ethoxy } ethanol:
Z- 1 – {4-[2-(2-Benzyloxy-ethoxy)ethoxy]phenyl} -4-chloro- 1 ,2-diphenyl -but- 1-ene (3.8 g, 7.4 mmol) is dissolved in ethyl acetate under nitrogen atmosphere , Zn powder (0.12 g, 1.85 mmol) and acetyl chloride (1.27 g, 16.3 mmol) are added and the mixture is stirred at 50 °C for 3 h (Bhar, 1995). The reaction mixture is cooled to room temperature, water (10 ml) is added and stirring is continued for additional 10 min. The aqueous layer is separated and the organic phase is washed with 1 M aqueous hydrogen chloride solution and with water. Ethyl acetate is evaporated and the residue is dissolved in methanol (16 ml) and water (4 ml). The acetate ester of the product is hydrolysed by making the mixture alkaline with sodium hydroxide (1 g) and stirring the mixture at room temperature for 1 h. Methanol is evaporated, water is added and the residue is extracted in ethyl acetate and washed with 1 M hydrogen chloride solution and with water. Ethyl acetate is evaporated and the residue is dissolved in toluene (25 ml), silica gel (0.25 g) is added and mixture is stirred for 15 min. Toluene is filtered and evaporated to dryness. The residue is crystallised from heptane-ethyl acetate (2:1). The yield is 71 %.
Z-isomer: 1H NMR (CDCI3): 2.92 (t, 2H), 3.41 (t, 2H), 3.58-3.63 (m, 2H), 3.69-3.80 (m, 4H), 3.96-4.01 (m, 2H), 6.56 (d, 2H), 6.78 (d, 2H), 7.10-7.40 (m, 10H).
E-2- {2- [4-(4-Chloro- 1 ,2-diphenyl-but- 1 -enyl)phenoxy]ethoxy} ethanol is prepared analogously starting from E-l-{4-[2-(2-benzyloxy- ethoxy)ethoxy]phenyl} -4-chloro- 1,2-diphenyl-but-l-ene. The product is purified by flash chromatography with toluene-methanol (10:0.5) as eluent.
E-isomer: 1H NMR (CDCI3): 2.97 (t, 2H), 3.43 (t, 2H), 3.65-3.79 (m, 4H), 3.85-3.90 (m, 2H), 4.13-4.17 (m, 2H), 6.85-7.25 (m, 2H).
Debenzylation of 1 – {4-[2-(2-benzyloxy-ethoxy)ethoxy]phenyl} -4-chloro- 1 ,2- diphenyl-but- 1-ene is also carried out by hydrogenation with Pd on carbon as a catalyst in ethyl acetate-ethanol solution at room temperature.
………….
PATENT
http://www.google.com/patents/US5491173

| Patent | Submitted | Granted |
|---|---|---|
| Method for the preparation of 2-{2-[4-(4-chloro-1,2-diphenylbut-1-enyl)phenoxy]ethoxy}ethanol and its isomers [US6891070] | 2004-06-17 | 2005-05-10 |
| Formulations of fispemifene [US2007104743] | 2007-05-10 | |
| METHODS FOR THE PREPARATION OF FISPEMIFENE FROM OSPEMIFENE [US7504530] | 2008-09-04 | 2009-03-17 |
| METHOD FOR THE PREPARATION OF THERAPEUTICALLY VALUABLE TRIPHENYLBUTENE DERIVATIVES [US2011015448] | 2011-01-20 | |
| METHOD FOR THE PREPARATION OF THERAPEUTICALLY VALUABLE TRIPHENYLBUTENE DERIVATIVES [US7812197] | 2008-08-28 | 2010-10-12 |
| WO2001036360A1 | 1 Nov 2000 | 25 May 2001 | Pirkko Haerkoenen | Triphenylalkene derivatives and their use as selective estrogen receptor modulators |
| EP0095875A2 | 20 May 1983 | 7 Dec 1983 | Farmos Group Ltd. | Novel tri-phenyl alkane and alkene derivatives and their preparation and use |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| WO2008099059A1 * | 13 Feb 2008 | 21 Aug 2008 | Hormos Medical Ltd | Method for the preparation of therapeutically valuable triphenylbutene derivatives |
| WO2008099060A2 * | 13 Feb 2008 | 21 Aug 2008 | Hormos Medical Ltd | Methods for the preparation of fispemifene from ospemifene |
| CN101636372B | 13 Feb 2008 | 27 Mar 2013 | 霍尔莫斯医疗有限公司 | Method for the preparation of therapeutically valuable triphenylbutene derivatives |
| EP1636159A1 * | 5 May 2004 | 22 Mar 2006 | Hormos Medical Ltd. | Method for the treatment or prevention of lower urinary tract symptoms |
| EP2518039A1 | 13 Feb 2008 | 31 Oct 2012 | Hormos Medical Ltd. | Method for the preparation of therapeutically valuable triphenylbutene derivatives |
| EP2821385A2 | 13 Feb 2008 | 7 Jan 2015 | Hormos Medical Ltd. | Method for the preparation of therapeutically valuable triphenylbutene derivatives |
| US7504530 | 13 Feb 2008 | 17 Mar 2009 | Hormos Medical Ltd. | Methods for the preparation of fispemifene from ospemifene |
| US7812197 | 13 Feb 2008 | 12 Oct 2010 | Hormos Medical Ltd. | Method for the preparation of therapeutically valuable triphenylbutene derivatives |
| US8293947 | 16 Sep 2010 | 23 Oct 2012 | Hormos Medical Ltd. | Method for the preparation of therapeutically valuable triphenylbutene derivatives |
| US8962693 | 19 Aug 2013 | 24 Feb 2015 | Hormos Medical Ltd. | Method for the treatment or prevention of lower urinary tract symptoms |
| WO2002090305A1 | Mar 21, 2002 | Nov 14, 2002 | Hormos Medical Corp | A new method for the preparation of 2-{2-[4-(4-chloro-1,2-diphenylbut-1-enyl)phenoxy]ethoxy}ethanol and its isomers |
| WO2004108645A1 | May 5, 2004 | Dec 16, 2004 | Hormos Medical Corp | Method for the treatment or prevention of lower urinary tract symptoms |
| WO2006024689A1 * | Jul 20, 2005 | Mar 9, 2006 | Taru Blom | Use of a selective estrogen receptor modulator for the manufacture of a pharmaceutical preparation for use in a method for the treatment or prevention of androgen deficiency |
| WO2007099410A2 * | Nov 9, 2006 | Sep 7, 2007 | Hormos Medical Ltd | Formulations of fispemifene |
| WO2014060640A1 | Oct 17, 2013 | Apr 24, 2014 | Fermion Oy | A process for the preparation of ospemifene |
| CN100526277C | May 5, 2004 | Aug 12, 2009 | 霍尔莫斯医疗有限公司 | Method for the treatment or prevention of lower urinary tract symptoms |
| CN102532073A * | Dec 30, 2011 | Jul 4, 2012 | 北京赛林泰医药技术有限公司 | Ethylene derivative serving as selective estrogen receptor modulators (SERMs) |
| EP1786408A1 * | Jul 20, 2005 | May 23, 2007 | Hormos Medical Ltd. | Use of a selective estrogen receptor modulator for the manufacture of a pharmaceutical preparation for use in a method for the treatment or prevention of androgen deficiency |
| EP1951250A2 * | Nov 22, 2006 | Aug 6, 2008 | SmithKline Beecham Corporation | Chemical compounds |
| EP2258360A2 | May 5, 2004 | Dec 8, 2010 | Hormos Medical Ltd. | Method for the treatment or prevention of lower urinary tract symptoms |
| EP2518039A1 | Feb 13, 2008 | Oct 31, 2012 | Hormos Medical Ltd. | Method for the preparation of therapeutically valuable triphenylbutene derivatives |
| EP2821385A2 | Feb 13, 2008 | Jan 7, 2015 | Hormos Medical Ltd. | Method for the preparation of therapeutically valuable triphenylbutene derivatives |
| US6891070 | Mar 21, 2002 | May 10, 2005 | Hormos Medical Corporation | Method for the preparation of 2-{2-[4-(4-chloro-1,2-diphenylbut-1-enyl)phenoxy]ethoxy}ethanol and its isomers |
| US7504530 | Feb 13, 2008 | Mar 17, 2009 | Hormos Medical Ltd. | Methods for the preparation of fispemifene from ospemifene |
| US7560589 | Jul 27, 2004 | Jul 14, 2009 | Smithkline Beecham Corporation | Cycloalkylidene compounds as modulators of estrogen receptor |
| US7569601 | May 14, 2007 | Aug 4, 2009 | Smithkline Beecham Corporation | Cycloalkylidene compounds as modulators of estrogen receptor |
| US7799828 | Jun 8, 2009 | Sep 21, 2010 | Glaxosmithkline Llc | Cycloalkylidene compounds as modulators of estrogen receptor |
| US7812197 | Feb 13, 2008 | Oct 12, 2010 | Hormos Medical Ltd. | Method for the preparation of therapeutically valuable triphenylbutene derivatives |
| US7825107 | May 22, 2007 | Nov 2, 2010 | Hormos Medical Ltd. | Method of treating men suffering from chronic nonbacterial prostatitis with SERM compounds or aromatase inhibitors |
| US8293947 | Sep 16, 2010 | Oct 23, 2012 | Hormos Medical Ltd. | Method for the preparation of therapeutically valuable triphenylbutene derivatives |
| US8299112 | Sep 15, 2011 | Oct 30, 2012 | Aragon Pharmaceuticals, Inc. | Estrogen receptor modulators and uses thereof |
| US8455534 | Sep 13, 2012 | Jun 4, 2013 | Aragon Pharmaceuticals, Inc. | Estrogen receptor modulators and uses thereof |
| US8962693 | Aug 19, 2013 | Feb 24, 2015 | Hormos Medical Ltd. | Method for the treatment or prevention of lower urinary tract symptoms |
| WO1996007402A1 * | Sep 6, 1995 | Mar 14, 1996 | Michael Degregorio | Triphenylethylenes for the prevention and treatment of osteoporosis |
| WO1996035417A1 * | May 10, 1996 | Nov 14, 1996 | Cancer Res Campaign Tech | Combinations of anti-oestrogen compounds and pkc modulators and their use in cancer therapy |
| WO1997032574A1 * | Mar 4, 1997 | Sep 12, 1997 | Degregorio Michael | Serum cholesterol lowering agent |
| WO1999042427A1 * | Feb 19, 1999 | Aug 26, 1999 | Kalapudas Arja | E-2-[4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy]ethanol and pharmaceutical compositions thereof |
| WO1999063974A2 * | Jun 10, 1999 | Dec 16, 1999 | Endorecherche Inc | Selective estrogen receptor modulator in combination with denydroepiandrosterone (dhea) or analogues |
| EP0095875A2 * | May 20, 1983 | Dec 7, 1983 | Farmos Group Ltd. | Novel tri-phenyl alkane and alkene derivatives and their preparation and use |
सुकून उतना ही देना प्रभू, जितने से जिंदगी चल जाये। औकात बस इतनी देना, कि औरों का भला हो जाये।
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
Join me on Facebook FACEBOOK
 amcrasto@gmail.com
amcrasto@gmail.com
 LIONEL MY SON
LIONEL MY SON
जिंदगी चल जाये।
औकात बस इतनी देना,
कि औरों का भला हो जाये।
///////

















Sorry, the comment form is closed at this time.