TERUTROBAN
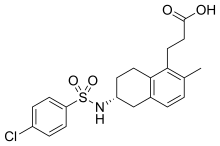
Terutroban is an antiplatelet agent developed by Servier Laboratories. as of|2008, it is tested for the secondaryprevention of acute thrombotic complications in the Phase III clinical trial PERFORM.
Method of action
Terutroban is a selective antagonist of the thromboxane receptor. It blocks thromboxane induced plateletaggregation and vasoconstriction.
Paper
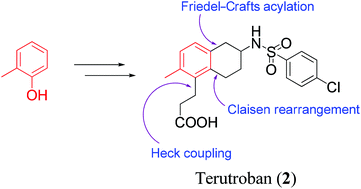
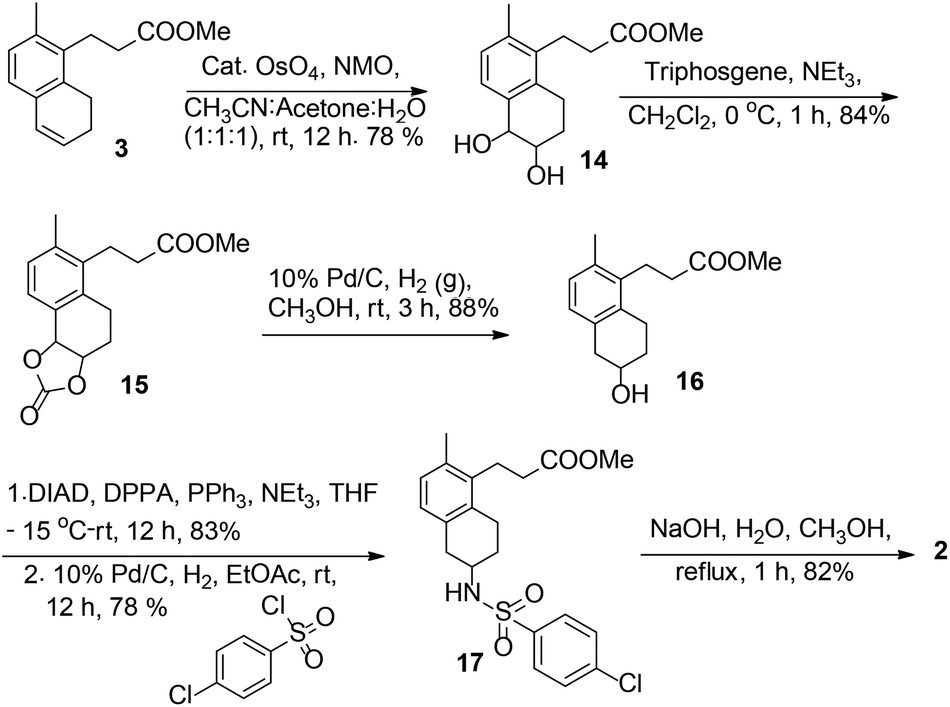
Total synthesis of a thromboxane receptor antagonist, terutroban
DOI: 10.1039/C4OB02302A, Paper
DOI: 10.1039/C4OB02302A
![3-[(6R)-6-[(4-chlorophenyl)sulfonylamino]-2-methyl-5,6,7,8-tetrahydronaphthalen-1-yl]propanoic acid NMR spectra analysis, Chemical CAS NO. 165538-40-9 NMR spectral analysis, 3-[(6R)-6-[(4-chlorophenyl)sulfonylamino]-2-methyl-5,6,7,8-tetrahydronaphthalen-1-yl]propanoic acid H-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-09/001/775/1775661_1h.png)
![3-[(6R)-6-[(4-chlorophenyl)sulfonylamino]-2-methyl-5,6,7,8-tetrahydronaphthalen-1-yl]propanoic acid NMR spectra analysis, Chemical CAS NO. 165538-40-9 NMR spectral analysis, 3-[(6R)-6-[(4-chlorophenyl)sulfonylamino]-2-methyl-5,6,7,8-tetrahydronaphthalen-1-yl]propanoic acid C-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-09/001/775/1775661_13c.png)
Terutroban is an antiplatelet agent developed by Servier Laboratories. It has been tested for the secondary prevention of acute thrombotic complications in the Phase III clinical trial PERFORM (Prevention of cerebrovascular and cardiovascular Events of ischemic origin with teRutroban in patients with a history oF ischemic strOke or tRansient ischeMic attack).[1] The study was prematurely stopped and thus it could not be determined whether terutroban has a better effect than aspirin.
Method of action
Terutroban is a selective antagonist of the thromboxane receptor. It blocks thromboxane induced platelet aggregation andvasoconstriction.[2][3]


…………………..

10.1358/dof.2006.031.10.1038241
Thromboxane A2 (TxA2) is an unstable metabolite of arachidonic acid formed by the cyclooxygenase pathway and released from activated platelets, monocytes and damaged vessel walls, causing irreversible platelet aggregation, vasoconstriction and smooth muscle cell proliferation. From efforts to discover novel compounds that could block the deleterious actions of TxA2, the 2-aminotetralin derivative terutroban sodium (S-18886) emerged as a potent, orally active, long-acting, selective antagonist of thromboxane (TP) receptors. The agent was able to inhibit TP agonist-induced platelet aggregation and vasoconstriction and was selected for further development as an antiplatelet and antithrombotic agent. Terutroban has been shown to be effective in animal models of thrombosis, atherosclerosis and diabetic nephropathy and is currently undergoing phase III development for the secondary prevention of acute thrombotic complications of atherosclerosis.
References
- Hennerici, M. G.; Bots, M. L.; Ford, I.; Laurent, S.; Touboul, P. J. (2010). “Rationale, design and population baseline characteristics of the PERFORM Vascular Project: an ancillary study of the Prevention of cerebrovascular and cardiovascular Events of ischemic origin with teRutroban in patients with a history oF ischemic strOke or tRansient ischeMic attack (PERFORM) trial”. Cardiovascular Drugs and Therapy24 (2): 175–80. doi:10.1007/s10557-010-6231-2. PMC 2887499. PMID 20490906. edit
- H. Spreitzer (January 29, 2007). “Neue Wirkstoffe – Terutroban”. Österreichische Apothekerzeitung (in German) (3/2007): 116.
- Sorbera, LA, Serradell, N, Bolos, J, Bayes, M (2006). “Terutroban sodium”. Drugs of the Future 31 (10): 867–873.doi:10.1358/dof.2006.031.10.1038241
 |
|
| Systematic (IUPAC) name | |
|---|---|
| 3-((6R)-6-{[(4-Chlorophenyl)sulfonyl]amido}-2-methyl-5,6,7,8-tetrahydronaphthalen-1-yl]propanoic acid | |
| Clinical data | |
| Legal status |
|
| Routes | Oral |
| Pharmacokinetic data | |
| Half-life | 6–10 hours |
| Identifiers | |
| CAS number | 165538-40-9 609340-89-8 (sodium salt) |
| ATC code | None |
| PubChem | CID 9938840 |
| ChemSpider | 8114465 |
| UNII | A6WX9391D8 |
| Chemical data | |
| Formula | C20H22ClNO4S |
| Molecular mass | 407.911 g/mol |

Chief Scientist & Head, Division of Natural Products Chemistry, CSIR- Indian Institute of Chemical Technology
Chandrasekhar obtained his Bachelor’s and Master’s degrees in 1982 and 1985 respectively, from Osmania University, Hyderabad and excelled in the same with distinction. He then joined A. V. Rama Rao’s group at CSIR–IICT and earned his doctorate in 1991, also from Osmania University. Between 1991 and 1994 he was associated with J. R. Falck (University of Texas Southwestern Medical Center) as a postdoctoral student. In 1994, Chandrasekhar joined his parent institute (CSIR–IICT) as a scientist
srivaric@gmail.com
READ………..http://www.currentscience.ac.in/Volumes/108/02/0160.pdf
)/Images/CSIRlogo.gif) |
Council of Scientific and Industrial Research Ministry of Science and Technology, Government of India |
)/Images/iictlogo.gif) |
CSIR-IICT CSIR-Indian Institute of Chemical Technology |
)/Images/iict70logo.gif)
 DR AV RAMA RAO
DR AV RAMA RAO Professor J. R. Falck
Professor J. R. Falck Professor L. F. Tietze
Professor L. F. Tietze
Srivari Chandrasekhar, senior scientist, Organic Chemistry Division, Indian Institute of Chemical Technology (IICT), has been conferred Fellow of Indian Academy of Sciences, Bangalore.
According to a press release here on Tuesday, Dr. Chandrasekhar has been conferred the honour for his significant contribution in organic chemistry and medicinal chemistry.
The major contributions include synthesis of complex natural products, especially of marine origin with anti-cancer and anti-depressant properties, green chemistry and automation chemistry to make large number of new chemicals.
He has produced 25 Ph.D. students and published more than 200 papers in international journals. He is also a fellow of National Academy of Sciences.
 India has achieved many prizes in 2014. Before the year ends IICT scientist Srivari Chandrasekhar has added one more prize, he wins Infosys Prize. The scientist who has made important contributions in potential drug developments. Srivari Chandrasekhar from CSIR-IICT , Hyderabad, was announced the winner of the Infosys Prize 2014 in Physical Sciences. The award includes a purse of Rs. 55 lakh, a 22 carat gold medal and citation. The award will be presented by The President on January 5 in Kolkata. The prize is awarded annually by the Infosys Foundation.
India has achieved many prizes in 2014. Before the year ends IICT scientist Srivari Chandrasekhar has added one more prize, he wins Infosys Prize. The scientist who has made important contributions in potential drug developments. Srivari Chandrasekhar from CSIR-IICT , Hyderabad, was announced the winner of the Infosys Prize 2014 in Physical Sciences. The award includes a purse of Rs. 55 lakh, a 22 carat gold medal and citation. The award will be presented by The President on January 5 in Kolkata. The prize is awarded annually by the Infosys Foundation.
He had won the CSIR Technology award-2014 along with his team member
Chandrasekhar’s current contribution is to develop a technology for manufacturing Misoprostal, an abortive drug also used in the treatment of ulcers. Now we can easily get rid of Ulcer.
He has successfully prepared some important drug molecules such as bedaquiline for multi-drug resistant TB, Galantamine for Alzheimer’s disease, Sertraline for treatment of depression, Nebivolol for hypertension and marine natural products such as Eribulin, Azumamide, Arenamide and Bengazole which are scarce to get from nature, with potent biological activities.
As he moves on achieving his target , he has made contributions in synthesizing complex and scarcely available natural products in the laboratory using easily available chemicals.
Chandrasekhar has over 250 publications in national and international journals to his credit.
Prof. Chandrasekhar has displayed an exceptional flair for identifying and synthesizing molecules of biological relevance, topical synthetic interest and utility to industry. His research efforts, with an impressive degree of innovations and enterprise, have led to the synthesis of complex and scarcely available natural products and new molecular entities for affordable healthcare. His endeavors have provided cost-effective technologies to chemical industry through identification of new reagents / solvents for specific transformations. Chandrasekhar’s group has synthesized several classes of complex natural products in optically pure form employing chiral pool precursors and catalytic asymmetric reactions and his syntheses of pladienolide, azumamide, bengazole etc., bear testimony to the efficacy of such approaches.
His passion and commitment to topical health related problems is through provisioning for better and affordable access to important drugs. Mention may be made of hissynthesis of bedaquiline, the first drug approved by FDA after a gap of over 40 years for the treatment of multi-drug resistant TB through simpler transformations and higher yields to ensure ready availability. He along with a team atIICT has developed a scalable synthetic route for misoprostol (a hormone like biologically important synthetic prostaglandin) used to prevent gastric ulcer, induce labor and / or abortion (particularly for safe termination of unwanted pregnancies), which has already been commercialized.