
NARINGENIN
Naringenin; CAS: 480-41-1
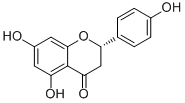 Aldrich Library of 13C and 1H FT NMR Spectra, 1992, 2, 915C
Aldrich Library of 13C and 1H FT NMR Spectra, 1992, 2, 915C

NARINGENIN

Naringenin; CAS: 480-41-1
 Aldrich Library of 13C and 1H FT NMR Spectra, 1992, 2, 915C
Aldrich Library of 13C and 1H FT NMR Spectra, 1992, 2, 915C

TERUTROBAN
Terutroban is an antiplatelet agent developed by Servier Laboratories. as of|2008, it is tested for the secondaryprevention of acute thrombotic complications in the Phase III clinical trial PERFORM.
Method of action
Terutroban is a selective antagonist of the thromboxane receptor. It blocks thromboxane induced plateletaggregation and vasoconstriction.
DOI: 10.1039/C4OB02302A
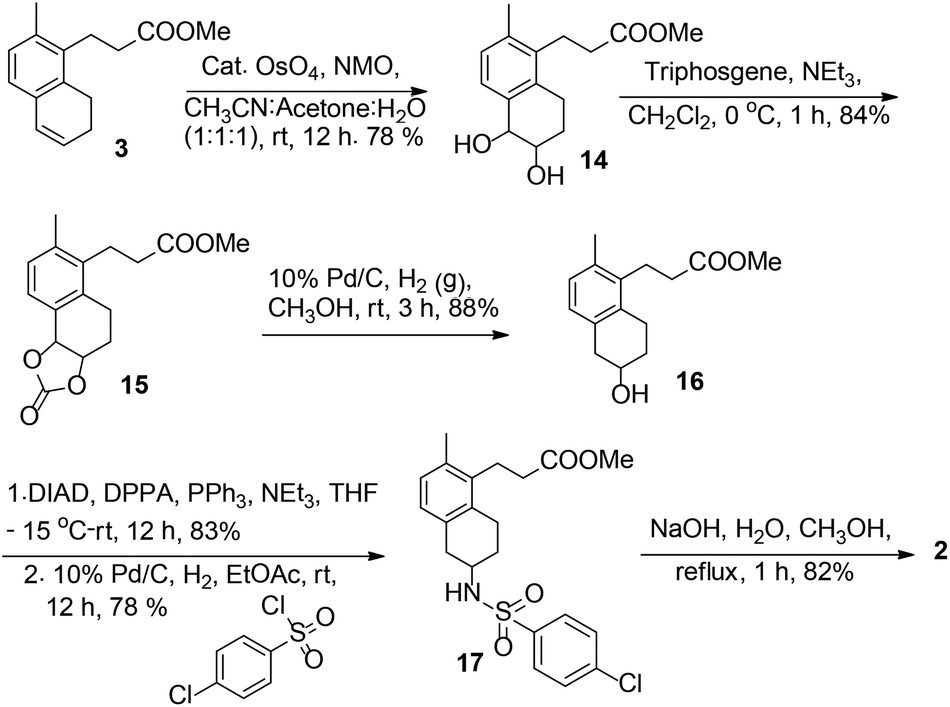
![3-[(6R)-6-[(4-chlorophenyl)sulfonylamino]-2-methyl-5,6,7,8-tetrahydronaphthalen-1-yl]propanoic acid NMR spectra analysis, Chemical CAS NO. 165538-40-9 NMR spectral analysis, 3-[(6R)-6-[(4-chlorophenyl)sulfonylamino]-2-methyl-5,6,7,8-tetrahydronaphthalen-1-yl]propanoic acid H-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-09/001/775/1775661_1h.png)
![3-[(6R)-6-[(4-chlorophenyl)sulfonylamino]-2-methyl-5,6,7,8-tetrahydronaphthalen-1-yl]propanoic acid NMR spectra analysis, Chemical CAS NO. 165538-40-9 NMR spectral analysis, 3-[(6R)-6-[(4-chlorophenyl)sulfonylamino]-2-methyl-5,6,7,8-tetrahydronaphthalen-1-yl]propanoic acid C-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-09/001/775/1775661_13c.png)
Terutroban is an antiplatelet agent developed by Servier Laboratories. It has been tested for the secondary prevention of acute thrombotic complications in the Phase III clinical trial PERFORM (Prevention of cerebrovascular and cardiovascular Events of ischemic origin with teRutroban in patients with a history oF ischemic strOke or tRansient ischeMic attack).[1] The study was prematurely stopped and thus it could not be determined whether terutroban has a better effect than aspirin.
Terutroban is a selective antagonist of the thromboxane receptor. It blocks thromboxane induced platelet aggregation andvasoconstriction.[2][3]


…………………..

10.1358/dof.2006.031.10.1038241
Thromboxane A2 (TxA2) is an unstable metabolite of arachidonic acid formed by the cyclooxygenase pathway and released from activated platelets, monocytes and damaged vessel walls, causing irreversible platelet aggregation, vasoconstriction and smooth muscle cell proliferation. From efforts to discover novel compounds that could block the deleterious actions of TxA2, the 2-aminotetralin derivative terutroban sodium (S-18886) emerged as a potent, orally active, long-acting, selective antagonist of thromboxane (TP) receptors. The agent was able to inhibit TP agonist-induced platelet aggregation and vasoconstriction and was selected for further development as an antiplatelet and antithrombotic agent. Terutroban has been shown to be effective in animal models of thrombosis, atherosclerosis and diabetic nephropathy and is currently undergoing phase III development for the secondary prevention of acute thrombotic complications of atherosclerosis.
 |
|
| Systematic (IUPAC) name | |
|---|---|
| 3-((6R)-6-{[(4-Chlorophenyl)sulfonyl]amido}-2-methyl-5,6,7,8-tetrahydronaphthalen-1-yl]propanoic acid | |
| Clinical data | |
| Legal status |
|
| Routes | Oral |
| Pharmacokinetic data | |
| Half-life | 6–10 hours |
| Identifiers | |
| CAS number | 165538-40-9 609340-89-8 (sodium salt) |
| ATC code | None |
| PubChem | CID 9938840 |
| ChemSpider | 8114465 |
| UNII | A6WX9391D8 |
| Chemical data | |
| Formula | C20H22ClNO4S |
| Molecular mass | 407.911 g/mol |

Chief Scientist & Head, Division of Natural Products Chemistry, CSIR- Indian Institute of Chemical Technology
Chandrasekhar obtained his Bachelor’s and Master’s degrees in 1982 and 1985 respectively, from Osmania University, Hyderabad and excelled in the same with distinction. He then joined A. V. Rama Rao’s group at CSIR–IICT and earned his doctorate in 1991, also from Osmania University. Between 1991 and 1994 he was associated with J. R. Falck (University of Texas Southwestern Medical Center) as a postdoctoral student. In 1994, Chandrasekhar joined his parent institute (CSIR–IICT) as a scientist
srivaric@gmail.com
READ………..http://www.currentscience.ac.in/Volumes/108/02/0160.pdf
)/Images/CSIRlogo.gif) |
Council of Scientific and Industrial Research Ministry of Science and Technology, Government of India |
)/Images/iictlogo.gif) |
CSIR-IICT CSIR-Indian Institute of Chemical Technology |
)/Images/iict70logo.gif)
 DR AV RAMA RAO
DR AV RAMA RAO Professor J. R. Falck
Professor J. R. Falck Professor L. F. Tietze
Professor L. F. Tietze
Srivari Chandrasekhar, senior scientist, Organic Chemistry Division, Indian Institute of Chemical Technology (IICT), has been conferred Fellow of Indian Academy of Sciences, Bangalore.
According to a press release here on Tuesday, Dr. Chandrasekhar has been conferred the honour for his significant contribution in organic chemistry and medicinal chemistry.
The major contributions include synthesis of complex natural products, especially of marine origin with anti-cancer and anti-depressant properties, green chemistry and automation chemistry to make large number of new chemicals.
He has produced 25 Ph.D. students and published more than 200 papers in international journals. He is also a fellow of National Academy of Sciences.
 India has achieved many prizes in 2014. Before the year ends IICT scientist Srivari Chandrasekhar has added one more prize, he wins Infosys Prize. The scientist who has made important contributions in potential drug developments. Srivari Chandrasekhar from CSIR-IICT , Hyderabad, was announced the winner of the Infosys Prize 2014 in Physical Sciences. The award includes a purse of Rs. 55 lakh, a 22 carat gold medal and citation. The award will be presented by The President on January 5 in Kolkata. The prize is awarded annually by the Infosys Foundation.
India has achieved many prizes in 2014. Before the year ends IICT scientist Srivari Chandrasekhar has added one more prize, he wins Infosys Prize. The scientist who has made important contributions in potential drug developments. Srivari Chandrasekhar from CSIR-IICT , Hyderabad, was announced the winner of the Infosys Prize 2014 in Physical Sciences. The award includes a purse of Rs. 55 lakh, a 22 carat gold medal and citation. The award will be presented by The President on January 5 in Kolkata. The prize is awarded annually by the Infosys Foundation.
He had won the CSIR Technology award-2014 along with his team member
Chandrasekhar’s current contribution is to develop a technology for manufacturing Misoprostal, an abortive drug also used in the treatment of ulcers. Now we can easily get rid of Ulcer.
He has successfully prepared some important drug molecules such as bedaquiline for multi-drug resistant TB, Galantamine for Alzheimer’s disease, Sertraline for treatment of depression, Nebivolol for hypertension and marine natural products such as Eribulin, Azumamide, Arenamide and Bengazole which are scarce to get from nature, with potent biological activities.
As he moves on achieving his target , he has made contributions in synthesizing complex and scarcely available natural products in the laboratory using easily available chemicals.
Chandrasekhar has over 250 publications in national and international journals to his credit.
Prof. Chandrasekhar has displayed an exceptional flair for identifying and synthesizing molecules of biological relevance, topical synthetic interest and utility to industry. His research efforts, with an impressive degree of innovations and enterprise, have led to the synthesis of complex and scarcely available natural products and new molecular entities for affordable healthcare. His endeavors have provided cost-effective technologies to chemical industry through identification of new reagents / solvents for specific transformations. Chandrasekhar’s group has synthesized several classes of complex natural products in optically pure form employing chiral pool precursors and catalytic asymmetric reactions and his syntheses of pladienolide, azumamide, bengazole etc., bear testimony to the efficacy of such approaches.
His passion and commitment to topical health related problems is through provisioning for better and affordable access to important drugs. Mention may be made of hissynthesis of bedaquiline, the first drug approved by FDA after a gap of over 40 years for the treatment of multi-drug resistant TB through simpler transformations and higher yields to ensure ready availability. He along with a team atIICT has developed a scalable synthetic route for misoprostol (a hormone like biologically important synthetic prostaglandin) used to prevent gastric ulcer, induce labor and / or abortion (particularly for safe termination of unwanted pregnancies), which has already been commercialized.
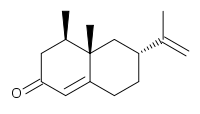
D. Srinivasa Reddy of CSIR-National Chemical Laboratory Pune devised (J. Org. Chem. 2013, 78, 8149. DOI: 10.1021/jo401033j) a cascade protocol of Diels-Alder cycloaddition of 8 to the diene 7 followed by intramolecular aldol condensation, to give the enone 9. Oxidative manipulation followed by methylenation completed the synthesis of the commercially important grapefruit flavor Nootkatone (10).

A simple and efficient synthesis of functionalized cis-hydrindanes and cis-decalins was achieved using a sequential Diels–Alder/aldol approach in a highly diastereoselective manner. The scope of this method was tested with a variety of substrates and was successfully applied to the synthesis of two natural products in racemic form. The highlights of the present work provide ready access to 13 new cis-hydrindanes/cis-decalins, a protecting group-free total synthesis of an insect repellent Nootkatone, and the first synthesis of a Noreremophilane using the shortest sequence.
(4R*,4aS*,6R*)-4,4a-Dimethyl-6-(prop-1-en-2-yl)-4,4a,5,6,7,8-hexahydronaph thaen-2(3H)-one ((±)-Nootkatone 20)
(±)-Nootkatone 20 (19 mg, 65%). IRυmax(film) 2923, 1668, 1606, 1459 cm–1; 1H NMR (400 MHz, CDCl3) δ 5.77 (s, 1 H), 4.74 (s, 1 H), 4.72(s, 1 H), 2.50 (ddt, J = 15.3, 5.0, 1.8 Hz, 1 H), 2.40–2.24 (m, 4 H), 2.04–1.89 (m, 3 H),1.74 (s, 3 H), 1.40–1.29 (m, 2 H), 1.11 (s, 3 H), 0.96 (d, J = 6.7 Hz, 3 H); 13C NMR (100 MHz, CDCl3) δ 199.9, 170.7, 149.3, 124.8, 109.4, 44.0, 42.2, 40.6, 40.5, 39.5, 33.2, 31.7, 21.0, 17.0, 15.0.
 |
|
| Names | |
|---|---|
| IUPAC name
4-α,5-Dimethyl-1,2,3,4,4α,5,6,7-octahydro-7-keto-3-isopropenylnaphthalene
|
|
| Other names
(+)-nootkatone
|
|
| Identifiers | |
| CAS number | 4674-50-4 |
| ChEMBL | ChEMBL446299 |
| ChemSpider | 1064812 |
| Jmol-3D images | Image |
| KEGG | C17914 |
| PubChem | 1268142 |
| Properties | |
| C15H22O | |
| Molar mass | 218.33 g·mol−1 |
| Appearance | Viscous yellow in its liquid form |
| Density | 0.968 g/mL |
| Melting point | 36 °C (97 °F; 309 K) |
| Boiling point | 170 °C (338 °F; 443 K) |
| Hazards | |
| S-phrases | S23 S24 S25 |
| Flash point | ~ 100 °C (212 °F) |
Nootkatone is a natural organic compound and is the most important and expensive aromatic of grapefruit.[1] It is a sesquiterpeneand a ketone.
Nootkatone was previouslythought to be one of the main chemical components of the smell and flavour of grapefruits. In its solid form it is usually found as crystals. As a liquid, it is viscous and yellow. Nootkatone is typically extracted from grapefruit, but can also be manufactured with genetically modified organisms, or through the chemical or biochemical oxidation of valencene. It is also found in Alaska yellow cedar trees[2] and vetiver grass.[3]
Nootkatone in spray form has been shown as an effective repellent/insecticide against deer ticks[3][4][5] and lone star ticks.[4][5] It is also an effective repellent/insecticide against mosquitos, and may repel bed bugs, head lice and other insects.[6] It is environmentally friendly insecticide, because it is a volatile essential oil that does not persist in the environment.[6] It is nontoxic to humans, is an approved food additive,[6] and “is commonly used in foods, cosmetics, and pharmaceuticals”.[3]
The CDC has licensed patents to two companies to produce an insecticide and an insect repellant.[6] Allylix, of San Diego, CA, is one of these licensees [7] and has developed an enzyme fermentation process that will produce nookatone more cost effectively.[8]

https://www.linkedin.com/pub/d-srinivasa-reddy-dsreddy/1/75a/139
Our group research interests are broadly in total synthesis of biologically active compounds and medicinal chemistry. Current projects include the total synthesis of bioactive natural products such as antiinflammatory agents, antibacterial agents, antimalarial compounds and anti-cancer agents. Targets are chosen for their interesting biological activity and moderate complexity, which drives our creative solutions to their synthesis. Our ability to achieve an efficient synthesis enables us to access sufficient quantities of target molecule for biological profiling and ready access to different analogs that may prove to be more selective and efficacious as a drug-like molecule. We have plans to divert our total synthesis projects into medicinal chemistry projects by simplifying the complex structures. In medicinal chemistry front, our main interest is to use “silicon-switch approach” to discover novel drugs or drug-like molecules with improved pharmacokintetic (PK) and pharmacodynamic (PD) properties.
DEC2014 NCL PUNE INDIA
DR ANTHONY WITH DR REDDY


Tomas Hudlicky
Department of Chemistry and Centre for Biotechnology, Brock University, 500 Glenridge Avenue, St. Catharines, Ontario L2S 3A1, Canada
E-mail: thudlicky@brocku.ca
Chemoenzymatic synthesis of complex natural and unnatural products: morphine, pancratistatin, and their analogs
Tomas Hudlicky
ARKIVOC 2006 (vii) 276-291
pp. 276 – 291
http://www.arkat-usa.org/get-file/23149/

Organic synthesis, biocatalysis, electrochemistry, asymmeric catalysis
Our group is engaged in a variety of projects ranging from total synthesis to investigations of new reactions and the design of enzyme inhibitors. In total synthesis, we work on implementing reliable and efficient routes to target molecules. Our ventures are exact and logical pursuits, yet serendipity, intuition, and art all form an integral part of designing a total synthesis.
We have exploited the biooxidation of aromatic compounds in an exhaustive approach to the synthetic design of carbohydrates and their derivatives. Our guiding principles are symmetry, simplicity, and precise order of operations so that any derivative or stereoisomer with a sugar backbone can be constructed. These products are tested for glycosidase inhibition, a process important in viral expression. In addition, carbocyclic sugars can act as cell messengers, and their availability through synthesis allows greater understanding of cellular communication. Oligomers of inositols can also be exploited in a rational design of templates for asymmetric synthesis and in the design of chiral polymers.
Morphine, pancratistatin, and taxol are other important molecules in which our group has invested much synthetic effort. Their total synthesis permits the investigation of new reactions and mechanistic pathways, which can then be applied in subsequent syntheses. Current effort is focused on designing a practical synthesis of morphine and analogs and in probing the active pharmacophore of pancratistatin in hopes of designing a more bio-available anti-tumor agent.
To address environmentally benign manufacturing, or Green Chemistry, we are exploiting organic electrochemistry as replacement technology for metal-based oxidizing and reducing agents.
Finally we are devoting some effort to studies in the mechanism of prokaryotic oxygenase enzymes. Our ultimate goal is the design of a synthetic enzyme mimic that can be used as a chiral reagent for aromatic cis-hydroxylation.
Research: organic synthesis, green chemistry, chemoenzymatic synthesis, biomanufacturing, biocatalysis
When people are trying to find Brock University they are often told to use the Schmon Tower, which can be seen throughout Niagara, as their guide. In the world of organic chemistry, Tomas Hudlicky, a Canada Research Chair in Biocatalysis, has earned the same sort of status.
The goal of Hudlicky’s research is the practical and efficient synthesis of new medicinal agents by asymmetric synthesis and total synthesis of natural products. His work related to the total synthesis of morphine and the anticancer drug pancratistatin is concerned with refinements and production of the alkaloids in a more efficient and environmentally benign manner. Analogs of both compounds are also being synthesized and evaluated for biological activities.
Hudlicky also conducts research in the area of organic electrochemistry, which provides “green” alternatives to oxidation and reduction methodology. His current research has led to several patent applications and licensing agreements with the Johnson & Johnson subsidiary Noramco. He has also developed a new, simpler route to Tamiflu, one of the few compounds effective against the illness known as H5N1 virus or bird flu.
Recognized as a “green” scientist, Hudlicky converts pharmaceutical waste into a variety of desirable pharmaceutical compounds. His research is responsible for giving the harmful waste of the past a new life as analgesic and anti-tumour products, specifically compounds used in the treatment of cancer, bio-infection and diabetes.
Hudlicky receives daily requests from across the globe to join his research team. The Cairns Family Health and Bioscience Research Complex will greatly improve the size and capacity of Hudlicky’s research facilities, allowing him to accept more graduate students to study with his group.
 |
|
| Systematic (IUPAC) name | |
|---|---|
| (1R,2S,3S,4S,4aR,11bR)-1,2,3,4,7-pentahydroxy-2,3,4,4a,5,11b-hexahydro-1H-[1,3]dioxolo[4,5-j]phenanthridin-6-one | |
| Clinical data | |
| Legal status |
?
|
| Identifiers | |
| CAS number | 96281-31-1 |
| ATC code | ? |
| PubChem | CID 441597 |
| ChemSpider | 390265 |
| Chemical data | |
| Formula | C14H15NO8 |
| Molecular mass | |
Pancratistatin (PST) is a natural compound initially extracted from Spider Lily,[1] a Hawaiian native plant, belonging to the familyAmaryllidaceae[2] (AMD).
Pancratistatin occurs naturally in Hawaiian Spider Lily, a flowering plant within the Amaryllidaceae family. Pancratistatin is mostly found in the bulb tissues of Spider Lilies. It has been shown that the enrichment of atmospheric CO2 can enhance the production ofantiviral secondary metabolites, including Pancratistatin, in these plants.[3] Pancratistatin can be isolated from the tropical bulbs ofHymenocallis littoralis in the order of 100 to 150 mg/kg when bulbs are obtained from the wild type in Hawaii. However, the compound has to be commercially extracted from field- and greenhouse-grown bulbs or from tissue cultures cultivated, for example, in Arizona, which generate lower levels of Pancratistatin (a maximum of 22 mg/kg) even in the peak month of October. After October, when the bulb becomes dormant, levels of Pancratistatin drop, down to only 4 mg/kg by May. Field-grown bulbs, which show monthly changes in Pancratistatin content, generate somewhat smaller amounts (2–5 mg/kg) compared to those grown in greenhouses cultivated over the same period.[4] There are about 40 different Spider Lily species worldwide and they are mainly native to theAndes of South America.
Pancratistatin is thought to have potential as a basis for the development of new pharmaceuticals,[5] particularly in the field of cancer treatment.[6]
Although there may not be a precise elucidation of Pancratistatin biological synthesis, there have been speculations on biosynthesis ofNarciclasine and Lycoricidine that are very similar to Pancratistatin in terms of structure. The biosynthesis is accomplished via synthesis from O-methylnorbelladine 4 by para-para phenol coupling to obtain vittatine 5 as an intermediate. Subsequent elimination of two carbon atoms and hydroxylations of compound 5 (vittatine) then leads to narciclasine.[7]
The first total synthesis of racemic (+/-) Pancratistatin was proposed by Samuel Danishefsky and Joung Yon Lee, which involved a very complex and long (40 steps) total synthesis. According to both Danishefsky and Joung, there were several weak steps in this synthesis that gave rise to a disappointing low synthetic yield. Amongst the most challenging issues, the Moffatt transposition and theorthoamide problem, which required a blocking maneuver to regiospecifically distinguish the C, hydroxyl group for rearrangement were considered to be the severe cases. However, both Danishevsky and Yon Lee stated that their approach towards the PST total synthesis was not out of merit and believed that their work would interest other medicinal scientists to construct a much more practical and efficient way for PST total synthesis.[8][9]
The work of Danishevsky and Joung provided the foundation for another total synthesis of PST, which was propounded by Li,M. in 2006. This method employed a more sophisticated approach, starting out with the pinitol 30 that its stereocenters are exactly the same as the ones in the C-ring of Pancratistatin.[10] Protection of the diol functions of compound 30 gave compound 31. The free hydroxyl of this was subsequently substituted by an azide to give 32. After removal of the silyl function, a cyclic sulfate was installed to obtain product 33. The Staudinger reaction gave the free amine 34 from azide 33. The coupling reaction between 34 and 35 gave compound 36 with a moderate yield. Methocymethyl protection of both the amide and the free phenol gave compound 37. Treatment of this latter product with t-BuLi followed by addition of cerium chloride gave compound 38. Full deprotection of 38 by BBr3 and methanol afforded pancratistatin 3 in 12 steps from commercially available pinitol with an overall yield of 2.3% 20.
a: TIPDSCl2, imidazole, DMAP, DMF, 24%. b: DMP, p-TsOH, acetone, 81%. c: PPh3, DEAD, CH3SO3H, CH2Cl2, 0 °C to r.t. then NaN3, DMF, 60 °C, 72%. d: TBAF, THF, 0 °C to r.t., 100%. e: SOCl2, Et3N, CH2Cl2, 0 °C. f: NaIO4, RuCl3, aq CH3CN, 87% (more than two steps). g: PPh3, aq THF, 0 °C to r.t., 94%. h: Et2O, 35, 0 °C, 64%. i: K2CO3, MOMCl,DMF, 84%. j: t-BuLi, CeCl3, ultrasound, THF, −78 °C to r.t., 72%. k: BBr3, CH2Cl2, −78 °C to 0 °C, 1 hour then MeOH, −78 °C to 0 °C, 2 hours, 52%.
A very recent approach to a stereocontrolled Pancratistatin synthesis was accomplished by Sanghee Kim from the National University of Seoul, in which claisen rearrangement of dihydropyranethlyene and a cyclic sulfate elimination reaction were employed 21. This reaction has proven to be very highly efficient as it produced an 83% overall synthetis yield. (Proved by H and 13C NMR).
The B ring of the phenanthridone (three membered nitrogen hetrocyclic ring) is formed using the Bischler-Napieralski reaction. The n precursor 3 with its stereocenters in the C ring is stereoselectively synthesized from the cis-disubstituted cyclohexene 4. The presence of unsaturated carbonyl in compound 4 suggested the use of a Claisen rearrangement of 3,4-dihydro-2H-pyranylethylene.[11]
The synthesis starts with the treatment of 6 with excess trimethyl phosphate. This reaction provides phosphate 7 in 97% yield. Using Honer-Wadsworth-Emmons reaction between 7 ands acrolein dimmer 8 in the presence of LHMDS in THF forms (E)-olefin 5 with very high stereoselectivity in 60% yield. Only less than 1% of (Z)-olefin was detected in the final product. The Claisen rearrangement of dihydropyranethylene forms the cis-distributed cyclohexene as a single isomer in 78% yield.
The next step of the synthesis involves the oxidation of aldehyde of compound 4 using NaClO2 to the corresponding carboxylic acid 9 in 90% yield. Iodolactonization of 9 and subsequent treatment with DBU in refluxing benzene gives rise to the bicyclic lacytone in 78% yield. Mthanolysis of lactone 10 with NaOMe forms a mixture of hydroxyl ester 11 and its C-4a epimer (Pancratistatin numbering). Saponification of the methyl ester 11 with LiOH was followed by a Curtius rearrangement of the resulting acid 12 with diphenylphosphoryl azide in refluxing toluene to afford isocyanate intermediate, which its treatment with NaOMe/MeOH forms the corresponding carbamate 13 in 82% yield.
The next steps of the synthesis involve the regioselevtive elimination of C-3 hydroxyl group and subsequent unsaturation achieved by cyclic sulfate elimination. Diol 16 needs to be treated with thionyl chloride and further oxidation with RuCl3 provides the cyclic sulfate 17 in 83% yield.[12] Treatment of cyclic sulfate with DBU yields the desired allylic alcohol 18 (67% yield).
Reaction with OsO4 forms the single isomerlization 19 in 88% yield. Peracetylation of 19 (77% yield) accompanied by Banwell’s modified Bischler-Napieralski forms the compound 20 with a little amount of isomer 21 ( 7:1 regioselectivity). The removal of protecting groups with NaOMe/MeOH forms Pancratistatin in 83%.
………………………………………………………….
Cheon-Gyu Cho of Hanyang University added (Org. Lett. 2013, 15, 5806. DOI: 10.1021/ol4028623) the activated dienophile 4 to the dienyl lactone to give, after oxidation, the dibromide 5. Debromination followed by oxidation led to the antineoplastic lactam Pancratistatin (6).
………………………………….
KAIXIN

MY EASTERN VENTURE TO PROPAGATE CHEMISTRY……………http://www.kaixin001.com/home/?_profileuid=159073878
 CHINA
CHINA
MY EASTERN VENTURE TO PROPAGATE CHEMISTRY……………http://www.kaixin001.com/home/?_profileuid=159073878
MY EASTERN VENTURE TO PROPAGATE CHEMISTRY……………http://www.kaixin001.com/home/?_profileuid=159073878
MY EASTERN VENTURE TO PROPAGATE CHEMISTRY……………http://www.kaixin001.com/home/?_profileuid=159073878\


: Hypertension or high blood pressure is one of the major independent risk factors for cardiovascular diseases. Angiotensin-I-converting enzyme (EC 3.4.15.1; ACE) plays an important physiological role in regulation of blood pressure by converting angiotensin I to angiotensin II, a potent vasoconstrictor. Therefore, the inhibition of ACE activity is a major target in the prevention of hypertension. Recently, the search for natural ACE inhibitors as alternatives to synthetic drugs is of great interest to prevent several side effects and a number of novel compounds such as bioactive peptides, chitooligosaccharide derivatives (COS) and phlorotannins have been derived from marine organisms as potential ACE inhibitors. These inhibitory derivatives can be developed as nutraceuticals and pharmaceuticals with potential to prevent hypertension. Hence, the aim of this review is to discuss the marine-derived ACE inhibitors and their future prospects as novel therapeutic drug candidates for treat hypertension.
– See more at: http://www.mdpi.com/1660-3397/8/4/1080/htm#sthash.B8fUm0Hw.dpuf
सुकून उतना ही देना प्रभू, जितने से
जिंदगी चल जाये।
औकात बस इतनी देना,
कि औरों का भला हो जाये।
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE

Vinita Gupta, 43, Group President and CEO, Lupin Pharmaceuticals and Director, Lupin
Feb 2015….India-based drugmaker Lupin has signed an agreement with Polish biopharmaceutical firm Celon Pharma to develop a fluticasone / salmeterol dry powder inhaler (DPI).
Under the deal, Lupin will take the responsibility for commercialisation of the product, which is a generic version of GlaxoSmithKline’s (GSK) Advair Diskus.
Lupin CEO Vinita Gupta said: “We are very pleased to partner with Celon given their experience in the development and manufacturing of fluticasone/salmeterol DPI in Europe…………..http://www.pharmaceutical-technology.com/news/newslupin-celon-pharma-partner-generic-version-gsks-advair-diskus-4514718?WT.mc_id=DN_News
Ms. Vinita Gupta is the CEO of Lupin Pharmaceuticals Inc, USA, (LPI) and Group President, Director on the board of Lupin Limited and a Director on the Board of Lupin’s Japanese subsidiary Kyowa Pharmaceuticals. Ms. Gupta is responsible for the North American and European business of the company.
Ms. Gupta joined Lupin in 1992 and developed Lupin’s entry strategy into US and Europe. Under her leadership Lupin has emerged as a leader in the US generic market as well as the only company from India to have a successful brand business in the US. As part of her responsibility she built the entire management team for the US and European business and supervised the development of the company’s pipeline.
Ms. Gupta holds a Bachelor’s degree in Pharmacy from the University of Mumbai and MBA from J L Kellogg Graduate School of Management, Northwestern University.
“A good year” is how Vinita Gupta, Group President and CEO at Lupin Pharmaceuticals, describes her company’s performance at a time when unsettling news was the key takeaway for pharma companies. Lupin grew by an impressive 35.9 per cent globally and 24 per cent in India. New product launches helped it grow its generics business by 52 per cent, making it the sixth-largest generics pharmaceutical company globally by market capitalisation and the third-largest Indian pharmaceutical company by revenues. “I can’t think of any challenges that affected Lupin’s performance during the last fiscal year,” says Gupta, 44. The company’s strategy now is to focus strongly on building its branded business globally.

Vinita Gupta, 43, Group President and CEO, Lupin Pharmaceuticals and Director, Lupin, is based in the United States, but has been in India a lot in the past one year.

With an expanding role in Lupin’s universe, Vinita has been spending more time outside the US, at times taking her six-year-old son, Krish with her. “He is getting exposure at a much younger age,” she says. Gupta herself was exposed to business at the age of 11 by her father Desh Bandhu Gupta, Lupin’s founder and Chairman.
“We almost had a family board at home, discussing work,” she says. Currently work goes well indeed, with Gupta taking new initiatives in India and also making the business more global. “I am focusing on drivers for growth in our business for the next five years,” she says.
Gupta is married to US-based businessman Brij Sharma.
She was 13 when she travelled to Switzerland with her father, to watch him position the family-run Lupin Limited, to negotiate and to strategise. It was enough to get her hooked. Enough for her to move away from a childhood fascination for art and enter the world of pharmaceuticals. Group president and CEO, Lupin Pharmaceuticals, and director on the board of Lupin Limited, Vinita Gupta has never regretted that decision. The 41-year-old is responsible for creating a substantial international presence for the company that was born in Mumbai in 1968 and named after a leguminous flower.
The Lupin group produces affordable generic and branded formulations in the world with a significant presence in cardiovasculars, diabetes, asthma, pediatrics and anti-infectives. But Desh Bandhu Gupta, her father, wanted the company to make an impact in the western market.
It was a challenge that seemed perfect for Gupta who graduated in pharmacy from the University of Mumbai and then spent a year working at the company in Mumbai. She then moved to the US for an MBA from the J.L. Kellogg Graduate School of Management at Northwestern University, following it with a brief stint in a US pharma company. But she didn’t want to be a mere “cog in the wheel”, returning to India to take up that initial challenge-to create a business strategy that would allow Lupin to enter American and European markets.
Today, Lupin is the ninth largest generics company in the US. It is also one of India’s top five pharmaceutical players and one of the fastest growing top 10 generic players in Japan and South Africa. The US arm of the business, Gupta’s baby, contributes to over 30 per cent of Lupin’s revenues, a company that clocked in close to Rs.4,000 crore in 2008-2009. Nine out of the 23 generic products Lupin has in the US market are at the number one position giving consistent competition to larger US pharma companies.

With brother NileshThe beginning however was difficult. After all, India wasn’t very well known in the US market. “We realised that we had the aspirations but not the infrastructure in the form of facilities to meet US and European requirements and standards,” she remembers. So she spent three years building the infrastructure, creating a process that would be acceptable to these regulated markets. The break came with Suprax, a pediatric antiinfective drug that was valued at nearly $60 million in the US market. Gupta had already filed for a generic of the brand. “Suddenly, we had the opportunity to brand the generic, so we licensed the brand name from the innovator as he had left the market,” she remembers. It was a three-person team with 40 outsourced sales people.
Today, the product’s sales are at $74 million. It has been satisfying, she says. “The innovator was in the market with a sales force of 300 people. We are 60.” The aim, she says, has always been to balance branded products as well as generic. The success, her father and chairman of the company, Desh Bandhu Gupta, says, stems not just from her determination. “It’s also her intimate understanding of the entire pharma spectrum with the motivation to see it through,” he says.
This determination became obvious when she managed to persuade the dean at Kellogg to give her admission, even though she was 19 and perhaps the youngest in her class. It was a challenging time as she learned to balance her work and household chores. “At that time in India, everything was handwritten. I had to do every single thing using the computer,” says Gupta who often bribed her friends with homemade Indian food to type out her projects. It taught her to be independent.
But it was perhaps, two months ago, when Gupta a bigger challenge. A deal that made tough seem an understatement. For Antara, an anti-cholesterol drug. “It was very much like Suprax, that was serendipity,” says Gupta. It was a large product with high potential. But the company was in bankruptcy. “I was sure we could do things differently with the product,” she says.
Gupta says Lupin was the first to file for the generic brand. But they couldn’t own the generic and the brand. She had six weeks to sell the generic, win the bid in the US bankruptcy court and buy the brand. She did it. At $38 million, one-third its market value. It’s a deal that Nilesh, her brother and group president and executive director, believes displayed his sister’s meticulous calibre. “There were three sets of negotiations going on at the same time. And while there were others involved, this deal was Vinita all the way,” he says.
Kamal Sharma, managing director, Lupin Limited, has watched Gupta transform from a teenager learning the ropes of a business to the successful go-getter that she is today. “She values, teaches and encourages her people to deliver consistent results year after year,” he says. It’s an attitude that is apparent from the get-go.

(L-R) With Richa, Kavita, Anuja and NileshAt the Trident, Mumbai, for the photo shoot, Gupta is comfortable surrounded by people, even though she is a little hesitant in front of the camera. It’s here that she actually seems to shed the image of an ultimate powerhouse, a businesswoman driven to succeed. It is here that she becomes the Mumbaikar who prefers a masala chai over brewed coffee and a plain tee over a designer label. In some ways, she is still the girl who grew up in a housing society near the airport in Vile Parle, Mumbai. It’s the kind of place where people still keep their doors open and where one can walk into a neighbour’s house without having to knock. Things weren’t handed to her on a golden platter, she admits. In fact, she says, their father taught them that, “as a family we would have to work harder to earn and deserve our right more than what other professionals do.”
As a child, she remembers sharing a room with her four siblings, Kavita, Anuja, Nilesh and Richa. She didn’t like that very much. “But now, when I think of it, I feel it was an amazing life,” she says. Her father adds that he always took his children to different countries, either on work or otherwise. It was his way of showing them the world and different experiences.
But a different side emerges as Gupta talks of the pharma industry. “I dreamed of taking what Dad had built and adding value to it in the western markets,” she says. “This is what I had always prepared myself for. I am living the dream.” And it isn’t as if there aren’t any downs. Six months ago, she remembers, the company received a warning letter from the Food and Drug Administration. She spent that time working to resolve their concerns. “And then three months later, we made one of our most attractive acquisitions. The industry is so quick changing, so dynamic. It always keeps you thinking,” she says.
For Nilesh though, Gupta is his sounding board, the eldest sister with whom he shares a relationship that complements their work profiles. And while Nilesh says with a laugh, Gupta doesn’t pull the bigsister act with him at work, home is a different story. Gupta admits with a mock sigh, “You can’t posture with your siblings. You can posture with anyone else, but not your siblings.”

With husband Brij and son KrishThe obvious downside, however, is family. Her work keeps her busy, sees her up and in office by 8 am, back just in time to spend about an hour with her four-year-old son Krish. “He was a very easy child till some time ago, but lately he has become very demanding,” she says with a smile. Just as Gupta was leaving her home in Baltimore, Maryland for her current trip to India, Krish demanded they go leaf-picking in their backyard. “More than anything, I loved watching the expression on his face while we were picking leaves. His smile brightens up my day,” she says.
As much as her job is a passion she tries to spend time with Krish and husband Brij Sharma, a businessman whom she met in the US. “My husband is a very good listener. I keep talking whenever I am with him and he listens even today,” she says with a laugh. A workout is a must, however, as Gupta heads to the gym every day, spinning the cycle even when she was eight months pregnant.
“My husband jokes that’s the reason why Krish thinks and behaves ahead of his age,” laughs Gupta. But biking near the waterfront with her son and spending time on her husband’s boat is an activity that wins hands down. As does time spent with her two sisters Anuja and Richa, who live in Chicago. While Anuja is a pediatric cardiologist, Anuja is into public health. They do plan vacations together, but she often discovers that her brother Nilesh refuses to talk to her over the weekend. “Probably because I always end up talking about work,” she says with a laugh. “It has become so much a part of our lives,” she says.

In June2012 , Vinita Gupta, CEO of Lupin Pharmaceuticals Inc, the Indian drug maker’s US unit, received the “Entrepreneur of the Year” award from Ernst & Young in the health services and technology category for Maryland state of the US. Over the past year, the US business of Lupin crossed the $500 million mark.

DB Gupta (centre) Chairman, Vinita Gupta (right) CEO and Nilesh Gupta

ARKIVOC Volume 2006
Part (v): Commemorative Issue in Honor of
Facilitator: Luba Ignatovich
Scientific Editor: Mikael Begtrup
2. The structure of Omeprazole in the solid state: a 13C and 15N NMR/CPMAS study (EL-1719AP)
Rosa M. Claramunt, Concepción López and José Elguero
Full Text: PDF (193K)
pp. 5 – 11
The structure of Omeprazole in the solid state: a 13C and 15N NMR/CPMAS study
Rosa M. Claramunt,a Concepción López,a and José Elguero b *
a Departamento de Química Orgánica y Bio-Orgánica, Facultad de Ciencias, UNED, Senda del Rey 9, E-28040 Madrid, Spain
b Instituto de Química Médica, CSIC, Juan de la Cierva, 3. E-28006 Madrid, Spain E-mail: iqmbe17@iqm.csic.es
To our friend Professor Edmunds Lukevics on his 70th anniversary
 Edmunds Lukevics
Edmunds Lukevics
Abstract
The 13C and 15N CPMAS spectra of a solid sample of Omeprazole have been recorded and all the signals assigned. The sample consists uniquely of the 6-methoxy tautomer. For analytical purposes, the signals of the other tautomer, the 5-methoxy one, were estimated from the data in solution (Magn. Reson. Chem. 2004, 42, 712).
Keywords: Omeprazole, NMR, 13C, 15N, CPMAS, tautomerism, benzimidazole
see at
http://www.arkat-usa.org/arkivoc-journal/browse-arkivoc/2006/5/graphical-abstracts/
http://www.arkat-usa.org/get-file/22955/
Edmunds LUKEVICS
(14.12.1936 – 21.11.2009)
 |
Professor Edmunds LUKEVICS Latvian Institute of Organic Synthesis, Head of the Laboratory of Organometallic ChemistryAizkraukles iela 21, Riga, LV-1006 Latvia
|
Born: December 14, 1936, Liepaja, Latvia
Departed: November 21, 2009, Riga, Latvia
Interests:
Main Research:
Development of methods for the synthesis of organosilicon and -germanium derivatives of furan, thiophene and nitrogen-containing heterocycles ; study of the influence of organosilicon ,-germanium and -tin substituents on the direction of substitution and addition reactions of furan and thiophene derivatives ; study of hydrosilylation and hydrogermylation reactions, synthesis and investigation of properties of penta- and hexacoordinated organosilicon and -germanium derivatives; application of alkenyl silanes and germanes in the synthesis of nitrogen-containing heterocycles; application of phase-transfer catalysis and ultrasonic irradiation in organometallic synthesis; synthesis of biologically active organosilicon and organogermanium compounds and studies of their properties.
Education:
Experience:
Latvian Institute of Organic Synthesis –
Honours and Awards:
Professional Activities:
Vice-chairman, Habilitation and Promotion Council (Chemistry), University of Latvia, 1998- 2009
Member of Editorial Board for:
Khimiya Geterotsikicheskikh. Soedinenii (Chemistry of Heterocyclic Compounds, Springer), 1980-1985; Editor-in-chief, 1985 – 2009
Proceedings of Latvian Academy of Sciences, 1982-1990
Latvian Journal of Chemistry, 1991 – 2009
Bioorganicheskaya Khimiya, 1989 – 1993
Applied Organometallic Chemistry, 1990 – 2009
Main Group Metal Chemistry, 1992 – 2009
Metal-Based Drugs, 1993 – 2003
Mendeleev Communications, 1994 – 2009
Advances in Heterocyclic Chemistry, 1994 – 2009
Silicon Chemistry, 2001-2007
Arkivoc, 2001 – 2009
Bioinorganic Chemistry and Applications, 2003 – 2006
Heterocyclic Communications, 2005 – 2009
Molecules, 2008 – 2009
Journal of Organic and Pharmaceutical Chemistry (Ukraine), 2009 – 2009
Memberships:
Lectures
Invited Lectures at Universities
Invited Lectures and Symposium’s Plenary Lectures:
Recent/Representative Publications:
Research Projects:
Hobbies:
Opera, Basketball, Mountains.
Edmunds LUKEVICS
|
Edmunds LUKEVICS Head of Laboratory of Organometallic Chemistry Latvian Institute of Organic Synthesis
Born: December 14, 1936, Liepaja, Latvia |
Interests in inventing:
Main invention:In the sphere of medicament synthesis:
In the sphere of the synthesis of agricultural chemicals:
Selection of patent documents:Totally: 104 authors’ certificates of USSR, 11 patents of Latvia, 3 patents of Germany, 3 patents of Canada, 3 patents of France, 3 patents of Italy, 1 patent of Japan, 1 patent of Switzerland, 4 patents of Great Britain, 5 patents of USA. Patents of Latvia:
|
|
Riga latvia
The building of the Brotherhood of Blackheads is one of the most iconic buildings of Old Riga (Vecrīga)

RIGA

RIGA

RIGA

RIGA

Cook in traditional latvian dress serving local food for tourists Riga Latvia

Here, we report a continuous flow protocol for the [3 + 2] cycloaddition of nitrones, in situ generated from oximes, into bicyclic isoxazolidines. This thermal process required very high temperatures to be efficient that were not easily reached in conventional reactors. A couple of examples are presented and in both the flow process showed a greater performance than the batch mode. The process intensification study allowed the generation of 120 g/h of a key pharmaceutical intermediate.
see
http://pubs.acs.org/doi/abs/10.1021/op500350y


