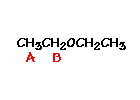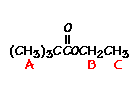Granulation is the act or process of forming or crystallizing into grains.[1] Granules typically have a size range between 0.2 to 4.0 mm depending on their subsequent use.
Synonym “Agglomeration”: Agglomeration processes or in a more general term particle size enlargement technologies are great tools to modify product properties. Agglomeration of powders is widely used to improve physical properties like: wettability, flowability, bulk density and product appearance.
Chemical industry
In the chemical industry, granulation refers to the act or process in which large objects are cut or shredded and remelted into granules or pellets.
Pharmaceutical industry
In the pharmaceutical industry, granulation refers to the act or process in which primary powder particles are made to adhere to form larger, multiparticle entities called granules. It is the process of collecting particles together by creating bonds between them. Bonds are formed by compression or by using a binding agent. Granulation is extensively used in the manufacturing of tablets and pellets (or spheroids).
The granulation process combines one or more powder particles and forms a granule that will allow tableting or spheronization process to be within required limits. This way predictable and repeatable process is possible and quality tablets or pellets can be produced using tabletting or spheronization equipment.
Purpose
Granulation is carried out for various reasons, one of those is to prevent the segregation of the constituents of powder mix. Segregation is due to differences in the size or density of the component of the mix. Normally, the smaller and/or denser particles tend to concentrate at the base of the container with the larger and/or less dense ones on the top. An ideal granulation will contain all the constituents of the mix in the correct proportion in each granule and segregation of granules will not occur.
Many powders, because of their small size, irregular shape or surface characteristics, are cohesive and do not flow well. Granules produced from such a cohesive system will be larger and more isodiametric, both factors contributing to improved flow properties.
Some powders are difficult to compact even if a readily compactable adhesive is included in the mix, but granules of the same powders are often more easily compacted. This is associated with the distribution of the adhesive within the granule and is a function of the method employed to produce the granule.
For example, if one were to make tablets from granulated sugar versus powdered sugar, powdered sugar would be difficult to compress into a tablet and granulated sugar would be easy to compress. Powdered sugar’s small particles have poor flow and compression characteristics. These small particles would have to be compressed very slowly for a long period of time to make a worthwhile tablet. Unless the powdered sugar is granulated, it could not efficiently be made into a tablet that has good tablet characteristics such as uniform content or consistent hardness.
Granulation techniques
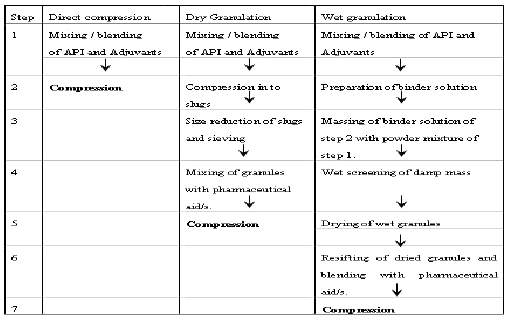
In pharmaceutical industry, two types of granulation technologies are employed, namely, wet granulation and dry granulation.
Wet granulation
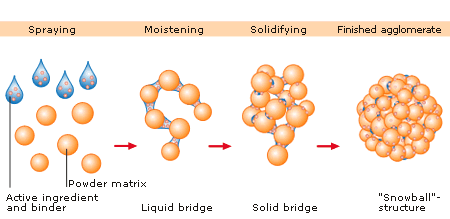
In wet granulation, granules are formed by the addition of a granulation liquid onto a powder bed which is under the influence of an impeller (in a High shear granulator, screws (in a twin screw granulator) [2] or air (in a fluidized bed granulator). The agitation resulting in the system along with the wetting of the components within the formulation results in the aggregation of the primary powder particles to produce wet granules.[2] The granulation liquid (fluid) contains a solvent which must be volatile so that it can be removed by drying, and be non-toxic. Typical liquids include water, ethanol and isopropanol either alone or in combination. The liquid solution can be either aqueous based or solvent based. Aqueous solutions have the advantage of being safer to deal with than solvents.
Water mixed into the powders can form bonds between powder particles that are strong enough to lock them together. However, once the water dries, the powders may fall apart. Therefore, water may not be strong enough to create and hold a bond. In such instances, a liquid solution that includes a binder (pharmaceutical glue) is required. Povidone, which is a polyvinyl pyrrolidone (PVP), is one of the most commonly used pharmaceutical binders. PVP is dissolved in water or solvent and added to the process. When PVP and a solvent/water are mixed with powders, PVP forms a bond with the powders during the process, and the solvent/water evaporates (dries). Once the solvent/water has been dried and the powders have formed a more densely held mass, then the granulation is milled. This process results in the formation of granules.
The process can be very simple or very complex depending on the characteristics of the powders, the final objective of tablet making, and the equipment that is available. In the traditional wet granulation method the wet mass is forced through a sieve to produce wet granules which is subsequently dried.
Dry granulation
The dry granulation process is used to form granules without using a liquid solution because the product granulated may be sensitive to moisture and heat. Forming granules without moisture requires compacting and densifying the powders. In this process the primary powder particles are aggregated under high pressure. Sweying granulator or high shear mixer-granulator can be used for the dry granulation.
Dry granulation can be conducted under two processes; either a large tablet (slug) is produced in a heavy duty tabletting press or the powder is squeezed between two counter-rotating rollers to produce a continuous sheet or ribbon of materials (roller compactor, commonly referred to as a chilsonator).
When a tablet press is used for dry granulation, the powders may not possess enough natural flow to feed the product uniformly into the die cavity, resulting in varying degrees of densification. The roller compactor (granulator-compactor) uses an auger-feed system that will consistently deliver powder uniformly between two pressure rollers. The powders are compacted into a ribbon or small pellets between these rollers and milled through a low-shear mill. When the product is compacted properly, then it can be passed through a mill and final blend before tablet compression.
See also
References
- Granulation definition
- ^ Jump up to:a b Dhenge, Ranjit M.; Washino, Kimiaki; Cartwright, James J.; Hounslow, Michael J.; Salman, Agba D. (2012). “Twin screw granulation using conveying screws: Effects of viscosity of granulation liquids and flow of powders”. Powder Technology. doi:10.1016/j.powtec.2012.05.045.
- Osborne, James; T. Althaus; L. Forny; G.Neideiretter; S.Palzer; M.Hounslow; A.D. Salman (2013). “Bonding Mechanisms Involved in the Roller Compaction of an Amorphous Material”.Chemical Engineering Science 86 (5th International Granulation Workshop): 61–69. doi:10.1016/j.ces.2012.05.012.
- Pharmaceutics – The science of dosage form design – M. E. Aulton 2nd EDT
- Pharmaceutical dosage forms and drug delivery system – Loyd V. Allen, Nicholas G. Popovich & Howard C. Ansel 8th EDT
- Lachman leon, Industrial pharmacy, special indian edition, CBS publishers
External links


READ
March 2014, Volume 9, Issue 1, pp 16-37
Closed-Loop Feedback Control of a Continuous Pharmaceutical Tablet Manufacturing Process via Wet Granulation
http://link.springer.com/article/10.1007/s12247-014-9170-9
Abstract
The wet granulation route of tablet manufacturing in a pharmaceutical manufacturing process is very common due to its numerous processing advantages such as enhanced powder flow and decreased segregation. However, this route is still operated in batch mode with little (if any) usage of an automatic control system. Tablet manufacturing via wet granulation, integrated with online/inline real time sensors and coupled with an automatic feedback control system, is highly desired for the transition of the pharmaceutical industry toward quality by design as opposed to quality by testing. In this manuscript, an efficient, plant-wide control strategy for an integrated continuous pharmaceutical tablet manufacturing process via wet granulation has been designed in silico. An effective controller parameter tuning strategy involving an integral of time absolute error method coupled with an optimization strategy has been used. The designed control system has been implemented in a flowsheet model that was simulated in gPROMS (Process System Enterprise) to evaluate its performance. The ability of the control system to reject the unknown disturbances and track the set point has been analyzed. Advanced techniques such as anti-windup and scale-up factor have been used to improve controller performance. Results demonstrate enhanced achievement of critical quality attributes under closed-loop operation, thus illustrating the potential of closed-loop feedback control in improving pharmaceutical tablet manufacturing operations.
……. CASE STUDY
http://www.madehow.com/Volume-4/Birth-Control-Pill.html
Oral contraceptives, or birth control pills, have been used by more than 60 million women worldwide, and are considered by many to be the most socially significant medical advance of the twentieth century. The birth control pill is a tablet taken daily by a woman to prevent pregnancy. The birth control pill does this by inhibiting the development of the egg in the woman’s ovary during her monthly menstrual cycle. During a woman’s menstrual cycle, a low estrogen level normally triggers the pituitary gland to send out a hormone that initiates development of an egg. The birth control pill releases enough synthetic estrogen to keep that hormone from being released during the monthly cycle.
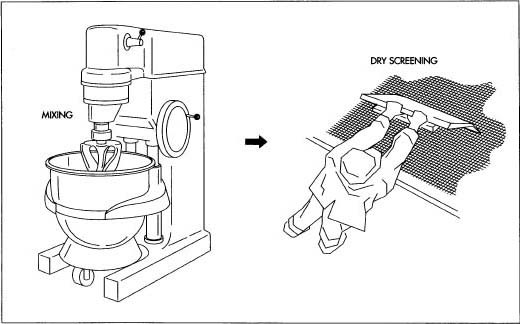
A part is pasted. article talks of manufacturing process
please click link
Read more: http://www.madehow.com/Volume-4/Birth-Control-Pill.html#ixzz38GpZ5xQX
Read more: http://www.madehow.com/Volume-4/Birth-Control-Pill.html#ixzz38GpVEWtL













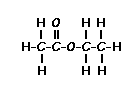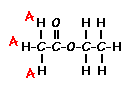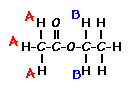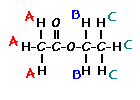
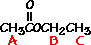





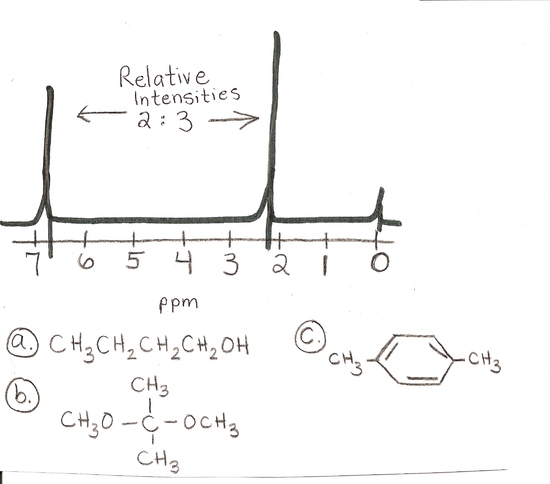
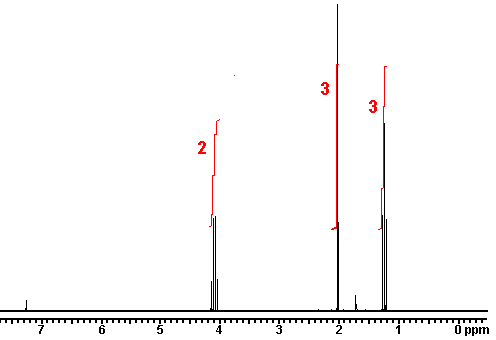
 answer is a and d
answer is a and d