
ALLOW SLIDESHARE TO LOAD…………
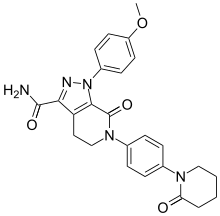
APIXABAN
Bristol-Myers Squibb and Pfizer announced that the U.S. Food and Drug Administration has accepted for review a Supplemental New Drug Application for Eliquis, for the prophylaxis of deep vein thrombosis, which may lead to pulmonary embolism, in patients who have undergone hip or knee replacement surgery.FULL STORY
Apixaban (INN, trade name Eliquis) is an anticoagulant for the prevention of venous thromboembolism and venous thromboembolic events. It is a direct factor Xa inhibitor. Apixaban has been available in Europe since May 2011 and was approved for preventing venous thromboembolism after elective hip or knee replacement. The FDA approved apixaban in December 2012 with an indication of reducing the risk of stroke and dangerous blood clots (systemic embolism) in patients with atrial fibrillation that is not caused by a heart valve problem. The drug was developed in a joint venture by Pfizer and Bristol-Myers Squibb

The challenge of delivering drugs to neurological targets has been a major roadblock to many promising therapies for treating diseases and disorders of the brain. The blood brain barrier blocks the vast majority of all small-molecules and virtually all large molecules from reaching therapeutic targets within the brain.FULL STORY