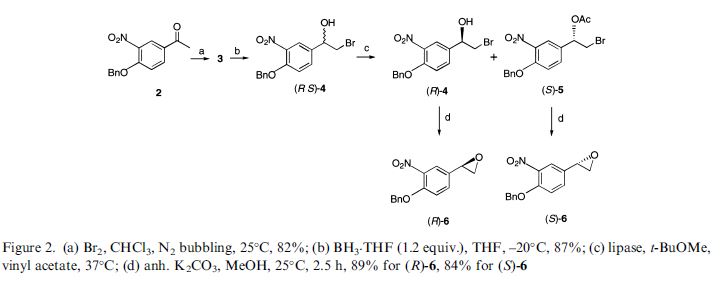
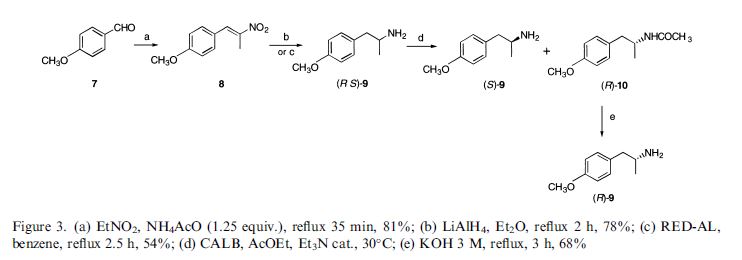
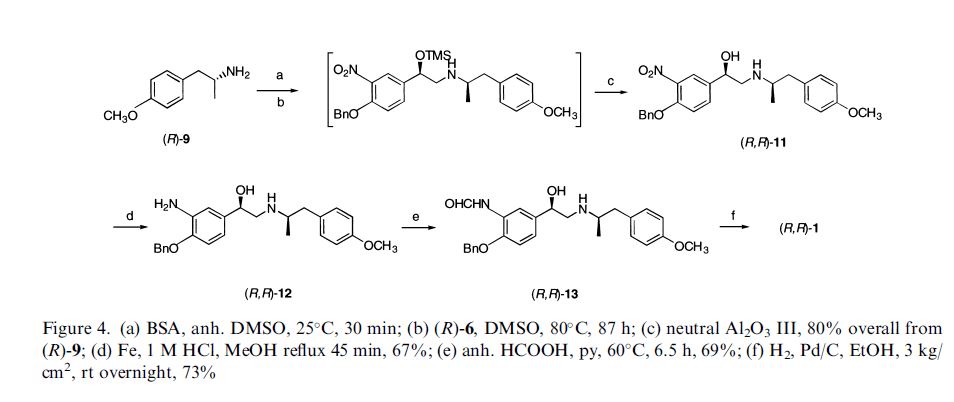
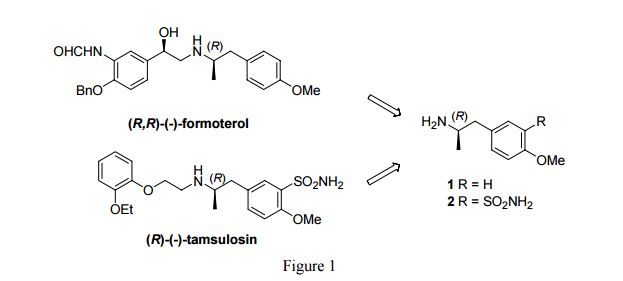
(R,R)-Formoterol
Cas 67346-49-0
Chronic obstructive pulmonary disease (COPD)
- Sunovion/Sepracor (Originator)
- Asthma Therapy, Bronchodilators, Chronic Obstructive Pulmonary Diseases (COPD), Treatment of, RESPIRATORY DRUGS, beta2-Adrenoceptor Agonists
- LAUNCHED 2007 , Phase III ASTHMA
Formamide, N-[2-hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]-


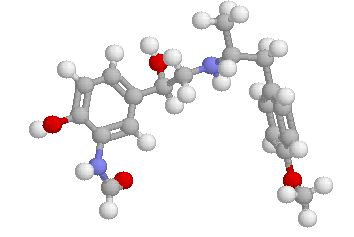
3D STRUCTURE
Arformoterol is a long-acting β2 adrenoreceptor agonist (LABA) indicated for the treatment of chronic obstructive pulmonary disease(COPD). It is sold by Sunovion, under the trade name Brovana, as a solution of arformoterol tartrate to be administered twice daily (morning and evening) by nebulization.[1]
Arformoterol inhalation solution, a long-acting beta2-adrenoceptor agonist, was launched in the U.S. in 2007 for the long-term twice-daily (morning and evening) treatment of bronchospasm in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. The product, known as Brovana(TM), for use by nebulization only, is the first long-acting beta2-agonist to be approved as an inhalation solution for use with a nebulizer. The product was developed and is being commercialized by Sunovion Pharmaceuticals (formerly Sepracor)
 Arformoterol ball-and-stick model
Arformoterol ball-and-stick model
Bronchodilators, in particular β2-adrenoceptor agonists, are recognized as very effective drugs to treat asthma and other bronchospastic conditions. Important characteristics for these drugs are activity, selectivity, duration of action, and onset. While the first-generation drugs (e.g., isoprenaline or terbutaline) were relatively unselective and short-acting, the current drugs have either a fast onset but only a short duration of action of about 4 h (albuterol) or a slow onset (20 min) with a longer duration of action (salmeterol). Formoterol (IUPAC name: 3-formamido-4-hydroxy-α-[[N-(p-methoxy-α-methylphenethyl)amino]methyl]benzyl alcohol) is unique in that it not only is extremely potent and selective but also has a duration of up to 12 h and a rapid onset of 1−5 min. Most β2-adrenoceptor agonists are currently marketed as racemates despite regulatory preference and different biological activity of pure enantiomers. In the case of formoterol it has been shown that the (R,R)-isomer is 1000 times more active than the (S,S)-isomer
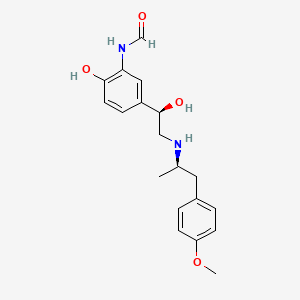
It is the active (R,R)-(−)-enantiomer of formoterol and was approved by the United States Food and Drug Administration (FDA) on October 6, 2006 for the treatment of COPD.
Arformoterol is a bronchodilator. It works by relaxing muscles in the airways to improve breathing. Arformoterol inhalation is used to prevent bronchoconstriction in people with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema. The use of arformoterol is pending revision due to safety concerns in regards to an increased risk of severe exacerbation of asthma symptoms, leading to hospitalization as well as death in some patients using long acting beta agonists for the treatment of asthma.
Arformoterol is an ADRENERGIC BETA-2 RECEPTOR AGONIST with a prolonged duration of action. It is used to manage ASTHMA and in the treatment of CHRONIC OBSTRUCTIVE PULMONARY DISEASE.
 Arformoterol (Brovana)
Arformoterol (Brovana)
Arformoterol is a beta2-Adrenergic Agonist. The mechanism of action of arformoterol is as an Adrenergic beta2-Agonist.
Arformoterol is a long-acting
beta-2 adrenergic agonist and isomer of
formoterol with bronchodilator activity. Arformoterol selectively binds to and activates
beta-2 adrenergic receptors in bronchiolar smooth muscle, thereby causing stimulation of adenyl cyclase, the enzyme that catalyzes the conversion of
adenosine triphosphate (
ATP) to cyclic-3′,5′-adenosine monophosphate (
cAMP). Increased intracellular
cAMP levels cause relaxation of bronchial smooth muscle and lead to a reduced release of inflammatory mediators from mast cells. This may eventually lead to an improvement of airway function.
Formoterol (Foradil) is a long acting β2-agonist used as a bronchodilator in the therapy of asthma and chronic bronchitis. The (R,R)-enantiomer has been shown to be more active than the other stereoisomers (R,S; S,R; and S,S) of formoterol. (R,R)-Formoterol is extremely potent and selective, having rapid onset (1−5 min) and long duration, and is 1000 times more active than the (S,S) isomer


Arformoterol tartrate
- Molecular FormulaC23H30N2O10
- Average mass494.492
- cas 200815-49-2
- 183-185°C
Butanedioic acid, 2,3-dihydroxy-, (2R,3R)-, compd. with formamide, N-[2-hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]- (1:1) [ACD/Index Name]
N-{2-hydroxy-5-[(1R)-1-hydroxy-2-{[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino}ethyl]phenyl}formamide 2,3-dihydroxybutanedioate (salt)
| N-[2-Hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino]�ethyl]phenyl]formamide (+)-(2R,3R)-Tartaric Acid; (-)-Formoterol 1,2-Dihydroxyethane-1,2-dicarboxylic Acid; (R,R)-Formoterol Threaric Acid; Arformoterol d-Tartaric Acid; Arformoterol d-α,β-Dihydroxysuccinic Acid |
(R,R)-Formoterol-L-(+)-tartrate
200815-49-2 CAS
Arformoterol tartrate (USAN)
Brovana
UNII:5P8VJ2I235
Arformoterol Tartrate, can be used in the synthesis of Omeprazole (O635000), which is a proton pump inhibitor, that inhibits gasteric secretion, also used in the treatment of dyspepsia, peptic ulcer disease, etc. Itis also the impurity of Esomeprazole Magnesium (E668300), which is the S-form of Omeprazole, and is a gastric proton-pump inhibitor. Also, It can be used for the preparation of olodaterol, a novel inhaled β2-adrenoceptor agonist with a 24h bronchodilatory efficacy.

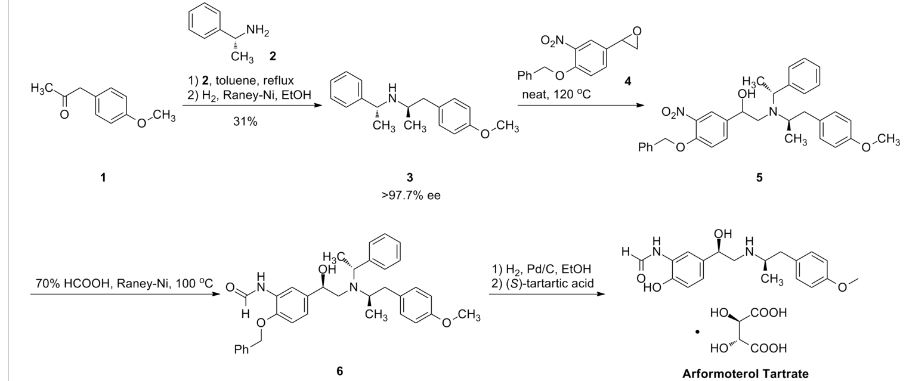
SYNTHESIS
PATENT
US-9309186
Example 1
Synthesis of (R,R)-Formoterol-L-tartrate Form D
A solution containing 3.9 g (26 mmol) of L-tartaric acid and 36 mL of methanol was added to a solution of 9 g (26 mmol) of arformoterol base and 144 mL methanol at 23.degree. C. Afterwards, the resulting mixture was seeded with form D and stirred at 23.degree. C. for 1 hour. It was then further cooled to 0-5.degree. C. for 1 hour and the product collected by filtration and dried under inlet air (atmospheric pressure) for 16 hours to provide 11.1 g (86% yield) (99.7% chemical purity, containing 0.14% of the degradation impurity (R)-1-(3-amino-4-hydroxyphenyl)-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethy- l]amino]ethanol) of (R,R)-formoterol L-tartrate form D, as an off white powder. .sup.1H-NMR (200 MHz, d.sub.6-DMSO) .delta.: 1.03 (d, 3H); 2.50-2.67 (m, 5H); 3.72 (s, 3H); 3.99 (s, 2H); 4.65-4.85 (m, 1H); 6.82-7.15 (m, 5H); 8.02 (s, 1H); 8.28 (s, 1H); 9.60 (s, NH). No residual solvent was detected (.sup.1H-NMR).
PSD: d.sub.50=2.3 .mu.m.
PAPER
Tetrahedron Letters, Vol. 38, No. 7, pp. 1125-1128, 1997
Enantio- and Diastereoselective Synthesis of all Four Stereoisomers of Formoterol
PAPER
Taking Advantage of Polymorphism To Effect an Impurity Removal: Development of a Thermodynamic Crystal Form of (R,R)-FormoterolTartrate
Chemical Research and Development, Sepracor Inc., 111 Locke Drive, Marlborough, Massachusetts 01752, U.S.A.
Org. Proc. Res. Dev., 2002, 6 (6), pp 855–862
DOI: 10.1021/op025531h
Abstract
The development and large-scale implementation of a novel technology utilizing polymorphic interconversion and crystalline intermediate formation of (R,R)-formoterol l-tartrate ((R,R)-FmTA, 1) as a tool for the removal of impurities from the final product and generation of the most thermodynamically stable crystal form is reported. The crude product was generated by precipitation of the free base as the l-tartrate salt in a unique polymorphic form, form B. Warming the resultant slurry effected the formation of a partially hydrated stable crystalline intermediate, form C, with a concomitant decrease in the impurity levels in the solid. Isolation and recrystallization of form C provided 1 in the thermodynamically most stable polymorph, form A.
SYN1
SYN 2
SYN 3
SYN 4
SYN 5
PATENT
Formoterol, (+/-)N-[2-hydroxy-5-[1-hydroxy-2-[[2-(p-methoxyphenyl)-2-propylamino]ethyl]phenyl]-formamide, is a highly potent and β2-selective adrenoceptor agonist having a long lasting bronchodilating effect when inhaled. Its chemical structure is depicted below:
Formoterol has two chiral centres, each of which can exist into two different configurations. This results into four different combinations, (R,R), (S,S), (S,R) and (R,S). Formoterol is commercially available as a racemic mixture of 2 diasteromers (R,R) and (S,S) in a 1:1 ratio. The generic name Formoterol always refers to its racemic mixture. Trofast et al. (Chirality, 1, 443, 1991) reported on the potency of these isomers, showing a decrease in the order of (R,R)>(R,S)≥(S,R)>(S,S). The (R,R) isomer, also known as Arformoterol, being 1000 fold more potent than the (S,S) isomer. Arformoterol is commercialised by Sepracor as Brovana
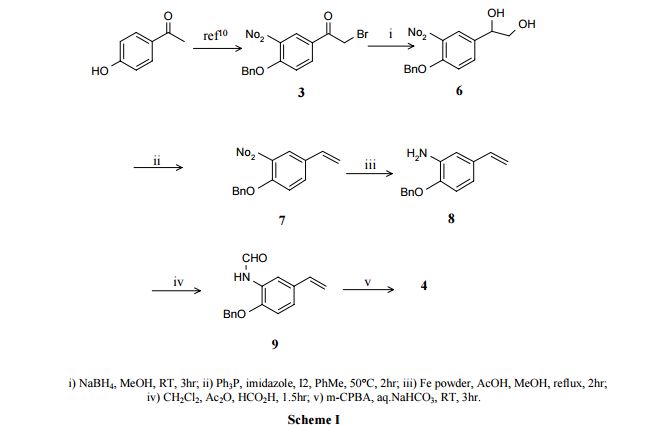
Formoterol was first disclosed in Japanese patent application (Application N° 13121 ) whereby Formoterol is synthesised by N-alkylation using a phenacyl bromide as described in the scheme below:
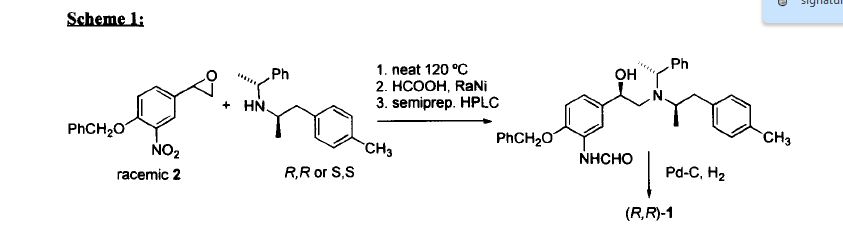
Afterwards, a small number of methods have been reported so far, regarding the synthesis of the (R,R) isomer, also referred as (R,R)-Formoterol and Arformoterol.
Murase et al. [Chem. Pharm. Bull. 26(4) 1123-1129(1978)] reported the preparation of (R,R)-Formoterol from a racemic mixture of the (R,R) and (S,S) isomers by optical resolution using optically active tartaric acid. Trofast et al. described a method in which 4-benzyloxy-3-nitrostyrene oxide was coupled with a optically pure (R,R)- or (S,S)-N-phenylethyl-N-(1-p-methoxyphenyl)-2-(propyl)amine to give a diastereomeric mixture of Formoterol precursors. These precursors were further separated by HPLC in order to obtain pure Formoterol isomers. Both synthetic processes undergo long synthetic procedures and low yields.
Patent publication
EP0938467 describes a method in which Arformoterol is prepared
via the reaction of the optically pure (R) N-benzyl-2-(4-methoxyphenyl)-1-(methylethylamine) with an optically pure (R)-4-benzyloxy-3-nitrostyrene oxide or (R)-4-benzyloxy-3-formamidostyrene oxide followed by formylation of the amino group. This method requires relatively severe reaction conditions, 24 h at a temperature of from 110 up to 130 °C as well as a further purification step using tartaric acid in order to eliminate diastereomer impurities formed during the process.
WO2009/147383 discloses a process for the preparation of intermediates of Formoterol and Arformoterol which comprises a reduction of a ketone intermediate of formula:
Using chiral reductive agent with an enantiomeric excess of about 98% which requires further purification steps to obtain a product of desired optical purity.
R,R)-Formoterol (Arformoterol) or a salt thereof from optically pure and stable intermediate (R)-2-(4-Benzyloxy-3-nitro-phenyl)-oxirane (compound II), suitable for industrial use, in combination with optically pure amine in higher yields, as depicted in the scheme below:
Compound (R, R)-1-(4-Benzyloxy-3-nitro-phenyl)-2-[[2-(4-methoxy-phenyl)-1-methylethyl]-(1-phenyl-ethyl)-amino]-ethanol (compound VI), having the configuration represented by the following formula:
Examples(R)-2-(4-Benzyloxy-3-nitro-phenyl)-oxirane (II)
A solution of 90 g (0.25 mol) of (R)-1-(4-Benzyloxy-3-nitro-phenyl)-2-bromo-ethanol (compound I) in 320 mL of toluene and 50 mL of MeOH was added to a stirred suspension of 46 g (0.33 mol) of K2CO3 in 130 mL of toluene and 130 mL of MeOH. The mixture was stirred at 40°C for 20 h and washed with water (400 mL). The organic phase was concentrated under reduced pressure to a volume of 100 mL and stirred at 25 °C for 30 min. It was then further cooled to 0-5°C for 30 min. and the product collected by filtration and dried at 40 °C to provide 67.1 g (97% yield) (98% chemical purity, 100% e.e.) of compound II as an off-white solid. 1 H-NMR (200 MHz, CDCl3) δ: 2.80-2.90 (m, 2H); 3.11-3.20 (m, 2H), 3.80-3.90 (m, 1H); 5.23 (s, 2H); 7.11 (d, 2H); 7.41 (m, 5H), 7.76 (d, 2H).
Preparation of (R,R)-[2-(4-Methoxy-phenyl)-1-methyl-ethyl]-(1-phenyl-ethyl)-amine (III)
A solution of 13 g (78.6 mmol) of 1-(4-Methoxy-phenyl)-propan-2-one and 8.3 g (78.6 mmol) of (R)-1-Phenylethylamine in 60 mL MeOH was hydrogenated in the presence of 1.7 g of Pt/C 5% at 10 atm. and 30 °C for 20 h. The mixture was filtered though a pad of diatomaceous earth and concentrated under reduced pressure to give compound III as an oil. The obtained oil was dissolved in 175 mL of acetone, followed by addition of 6.7 mL (80.9 mmol) of a 12M HCl solution. The mixture was stirred at 23 °C for 30 min and at 0-5 °C for 30 min. The product collected by filtration and dried at 40 °C to provide 13.8 g of the hydrochloride derivate as a white solid. The obtained solid was stirred in 100 mL of acetone at 23 °C for 1h and at 0-5 °C for 30 min, collected by filtration and dried at 40 °C to provide 13.2 g of the hydrochloride derivate as a white solid. This compound was dissolved in 100 mL of water and 100 mL of toluene followed by addition of 54 mL (54 mmol) of 1N NaOH solution. The organic phase was concentrated to give 11.7 g (55% yield) (99% chemical purity and 100% e.e) of compound III as an oil.1H-NMR (200 MHz, CDCl3) δ: 0.88 (d, 3H); 1.31 (d, 3H), 2.40-2.50 (m, 1H); 2.60-2.80 (m, 2H); 3.74 (s, 3H); 3.90-4.10 (m, 1H); 6.77- 6.98 (m, 4H), 7.31 (s, 5H).
Synthesis of (R,R)-1-(4-Benzyloxy-3-nitro-phenyl)-2-[[2-(4-methoxy-phenyl)-1-methyl-ethyl]-(1-phenyl-ethyl)-amino]-ethanol (IV)
A 1-liter flask was charged with 50g (0.18 mol) of II and 50g (0.18 mol) of III and stirred under nitrogen atmosphere at 140 °C for 20 h. To the hot mixture was added 200 mL of toluene to obtain a solution, which was washed with 200 mL of 1N HCl and 200 mL of water. The organic phase was concentrated under reduced pressure to give 99 g (99% yield) (88% chemical purity) of compound IV as an oil. Enantiomeric purity 100%. 1H-NMR (200 MHz, CDCl3) δ: 0.98 (d, 3H); 1.41 (d, 3H), 2.60-2.90 (m, 4H); 3.20-3.30 (m, 1H); 3.74 (s, 3H); 4.10-4.20 (m, 1H); 4.30-4.40 (m, 1H), 5.19 (s, 2H); 6.69-7.42 (m, 16H); 7.77 (s, 1H).
Synthesis of (R, R)-1-(3-Amino-4-benzyloxy-phenyl)-2-[[2-(4-methoxy-phenyl)-1-methyl-ethyl]-(1-phenyl-ethyl)-amino]-ethanol (V)
A solution of 99 g (0.18 mol) of IV in 270 mL IPA and 270 mL toluene was hydrogenated in the presence of 10 g of Ni-Raney at 18 atm and 40 °C for 20 h. The mixture was filtered though a pad of diatomaceous earth and the filtrate was concentrated under reduced pressure to give 87 g (92% yield) (83% chemical purity, 100 % e.e.) of compound V as an oil. 1H-NMR (200 MHz, CDCl3) δ: 0.97 (d, 3H); 1.44 (d, 3H), 2.60-2.90 (m, 4H); 3.20-3.30 (m, 1H); 3.74 (s, 3H); 4.10-4.20 (m, 1H); 4.30-4.40 (m, 1H), 5.07 (s, 2H); 6.67-6.84 (m, 7H); 7.25-7.42 (m, 10H).
Synthesis of (R,R)-N-(2-Benzyloxy-5-{1-hydroxy-2-[[2-(4-methoxy-phenyl)-1-methyl-ethyl]-(1-phenyl-ethyl)-amino]-ethyl)-phenyl)-formamide (VI)
24 mL (0.63 mol) of formic acid was added to 27 mL (0.28 mol) of acetic anhydride and stirred at 50 °C for 2 h under nitrogen atmosphere. The resulting mixture was diluted with 100 mL of CH2Cl2 and cooled to 0 °C. A solution of 78 g (0.15 mol) of V in 300 mL de CH2Cl2 was slowly added and stirred for 1h at 0 °C. Then, 150 mL of 10% K2CO3 aqueous solution were added and stirred at 0 °C for 15 min. The organic phase was washed twice with 400 mL of 10% K2CO3 aqueous solution and concentrated under reduced pressure to give 80 g (97% yield, 100% e.e.) (75% chemical purity) of compound VI as an oil. 1H-NMR (200 MHz, CDCl3) δ: 0.98 (d, 3H); 1.42 (d, 3H), 2.60-2.90 (m, 4H); 3.20-3.30 (m, 1H); 3.75 (s, 3H); 4.10-4.20 (m, 1H); 4.30-4.40 (m, 1H), 5.09 (s, 2H); 6.67-7.41 (m, 17H); 8.4 (d, 1H).
Synthesis (R,R)-N-(2-Hydroxy-5-{1-hydroxy-2-[2-(4-methoxy-phenyl)-1-methyl-ethylamino]-ethyl}-phenyl)-formamide (VII)
A solution of 8.5 g (16 mmol) of VI, previous purified by column chromatography on silica gel (AcOEt/heptane, 2:3), in 60 mL ethanol was hydrogenated in the presence of 0.14 g of Pd/C 5% at 10 atm. and 40 °C for 20 h. The mixture was filtered though a pad of diatomaceous earth and concentrated under reduced pressure to give 5 g (93% yield) (91% chemical purity, 100% e.e.) of compound VII as foam. m. p.= 58-60 °C. 1H-NMR (200 MHz, d6-DMSO) δ: 0.98 (d, 3H); 2.42-2.65 (m, 5H); 3.20-3.40 (m, 1H); 3.71 (s, 3H); 4.43-4.45 (m, 1H); 6.77-7.05 (m, 5H); 8.02 (s, 1H), 8.26 (s, 1H).
Synthesis (R,R)-N-(2-Hydroxy-5-{1-hydroxy-2-[2-(4-methoxy-phenyl)-1-methyl-ethylamino]-ethyl}-phenyl)-formamide (VII)
A solution of 46 g (0.08 mol) of VI, crude product, was dissolved in 460 mL ethanol and hydrogenated in the presence of 0.74 g of Pd/C 5% at 10 atm. and 40 ° C for 28 h. The mixture was filtered though a pad of diatomaceous earth and the filtrate was concentrated under reduced pressure to give 24 g (83% yield) (77% chemical purity, 100% e.e.) of compound VII as a foam. m. p. = 58-60 °C. 1H-NMR (200 MHz, d6-DMSO) δ: 0.98 (d, 3H); 2.42-2.65 (m, 5H); 3.20-3.40 (m, 1H); 3.71 (s, 3H); 4.43-4.45 (m, 1H); 6.77-7.05 (m, 5H); 8.02 (s, 1H), 8.26 (s, 1H).
The HPLC conditions used for the determination of the Chemical purity % are described in the table below:
-
|
| HPLC Column |
Kromasil 100 C-18 |
| Dimensions |
0.15 m x 4.6 mm x 5 µm |
| Buffer |
2.8 ml TEA (triethylamine) pH=3.00 H3PO4 (85%) in 1 L of H2O |
| Phase B |
Acetonitrile |
| Flow rate |
1.5 ml miN-1 |
| Temperature |
40 °C |
| Wavelength |
230 nm
The HPLC conditions used for the determination of the enantiomeric purity % are described in the table below: |
|
| HPLC Column |
Chiralpak AD-H |
| Dimensions |
0.25 m x 4.6 mm |
| Buffer |
n-hexane : IPA : DEA (diethyl amine) : H2O 85:15:0.1:0.1 |
| Flow rate |
0.8 ml min-1 |
| Temperature |
25 °C |
| Wavelength |
228 nm |

PATENT
Example 1
(R) -2- (4- benzyloxy-3-nitrophenyl) oxirane (I) (9. 86g, 36mmol) and (R) -I- (4- methoxy- phenyl) -N – [(R) -I- phenyl-ethyl] -2-amino-propane (II) (10. 8g, 40mmol) cast in the reaction flask, the reaction 20 hours at 140 ° C, the chiral Intermediate (III) (17. 3g, yield 88%). HPLC: de values of> 90%; MS (ESI) m / z: 541 3 (M ++ 1); 1H-NMR (CDCl3):.. Δ 0. 96 (d, 3H), 1 49 (d, 3H ), 2 · 15 (q, 1Η), 2 · 67 (dq, 2H), 2. 99 (dq, 2H), 3. 74 (s, 3H), 4. 09 (d, 1H), 4. 56 (q, 1H), 5. 24 (s, 2H), 6. 77 (dd, 4H), 7. 10 (d, 1H), 7. 25-7. 5 (m, 11H), 7. 84 ( s, 1H).
Example 2
(R) -2- (4- benzyloxy-3-nitrophenyl) oxirane (I) (9. 86g, 36mmol) and (R) -I- (4- methoxybenzene yl) -N – [(R) -I- phenyl-ethyl] -2-amino-propane (II) (10. 8g, 40mmol) and toluene 100ml, 110 ° C0-flow reactor 36 hours, the solvent was distilled off succeeded intermediates (III) (16. 8g, yield 85%).
Example 3
(R) -2- (4- benzyloxy-3-nitrophenyl) oxirane (I) (9. 86g, 36mmol) and (R) -I- (4- methoxybenzene After [(R) -I- phenyl-ethyl] -2-amino-propane (II) (10. 8g, 40mmol) and dichloromethane 100ml, 30 ° C for 48 hours, and the solvent was distilled off – yl) -N succeeded intermediates (III) (15. Sg, yield 80%).
Example 4
(R) -2- (4- benzyloxy-3-nitrophenyl) oxirane (I) (9. 86g, 36mmol) and (R) -I- (4- methoxybenzene yl) -N – [(R) -I- phenyl-ethyl] -2-amino-propane (II) (8. 75g, 32mmol) cast in the reaction flask, the reaction 20 hours at 140 ° C, the chiral intermediate form (III) (16. 3g, 83% yield).
Example 5
(R) -2- (4- benzyloxy-3-nitrophenyl) oxirane (I) (9. 86g, 36mmol) and (R) -I- (4- methoxybenzene yl) -N – [(R) -I- phenyl-ethyl] -2-amino-propane (II) (14. 6g, 54mmol) cast in the reaction flask, the reaction 20 hours at 140 ° C, the chiral intermediate form (III) (17. 5g, 89% yield).


Scheme
chirality 1991, 3, 443-50
Fumaric acid (0.138 mmol, 16 mg) was added to the residue dissolved in methanol. Evaporation of the solvent gave the
product (SS) W semifumarate (109 mg) characterized by ‘HNMR (4-D MSO) 6 (ppm) 1.00 (d, 3H, CHCH,), 4.624.70 (m, lH,
CHOH), 3.73 (s, 3H, OCH,), 6.M.9 (m, 3H, aromatic), 7.00 (dd,4H, aromatic), 6.49 (s, 1@ CH = CH fumarate). MS of disilylated
(SS) W: 473 (M +<H3,7%); 367 (M ‘<8H90, 45%); 310 61%). The (RSS) fraction was treated in the same manner
giving the product (R;S) W semifumarate, which was characterized by ‘H-NMR (4-DMSO) 6 (ppm) 1.01 (d, 3H, CHCH,),
3.76 (s, 3H, OC&), 6.49 (s, lH, CH=CH, fumarate) 6.M.9 (m, 3H, aromatic), 7.0 (dd, 4H, aromatic). MS of disilylated (R;S)
(M’X~~HIGNO1,7 %); 178 ( C I ~H~ ~N95O%,) ; 121 (CsH90, W. 473 (M’4H3, 5%); 367 (M’4gH90, 48%); 310
(M +–CI~HIGNO18, %); 178 (CIIHIGNO, 95%); 121 (CsH90, 52%). The structural data for the (RR) and (S;R) enantiomers
were in accordance with the proposed structures. The enantiomeric purity obtained for the enantiomers in each batch is
shown in Table 1.
Scheme

The enantioselective reduction of phenacyl bromide (I) with BH3.S(CH3)2 in THF catalyzed by the chiral borolidine (II) (obtained by reaction of (1R,2S)-1-amino-2-indanol (III) with BH3.S(CH3)2 in THF) gives the (R)-2-bromo-1-(4-benzyloxy-3-nitrophenyl)ethanol (IV), which is reduced with H2 over PtO2 in THF/toluene yielding the corresponding amino derivative (V). The reaction of (V) with formic acid and Ac2O affords the formamide (VI), which is condensed with the chiral (R)-N-benzyl-N-[2-(4-methoxyphenyl)-1-methylethyl]amine (VII) in THF/methanol providing the protected target compound (VIII). Finally, this compound is debenzylated by hydrogenation with H2 over Pd/C in ethanol. The intermediate the chiral (R)-N-benzyl-N-[2-(4-methoxyphenyl)-1-methylethyl]amine (VII) has been obtained by reductocondensation of 1-(4-methoxyphenyl)-2-propanone (IX) and benzylamine by hydrogenation with H2 over Pd/C in methanol yielding racemic N-benzyl-N-[2-(4-methoxyphenyl)-1-methylethyl]amine (X), which is submitted to optical resolution with (S)-mandelic acid to obtain the desired (R)-enantiomer (VII).
Org Process Res Dev1998,2,(2):96
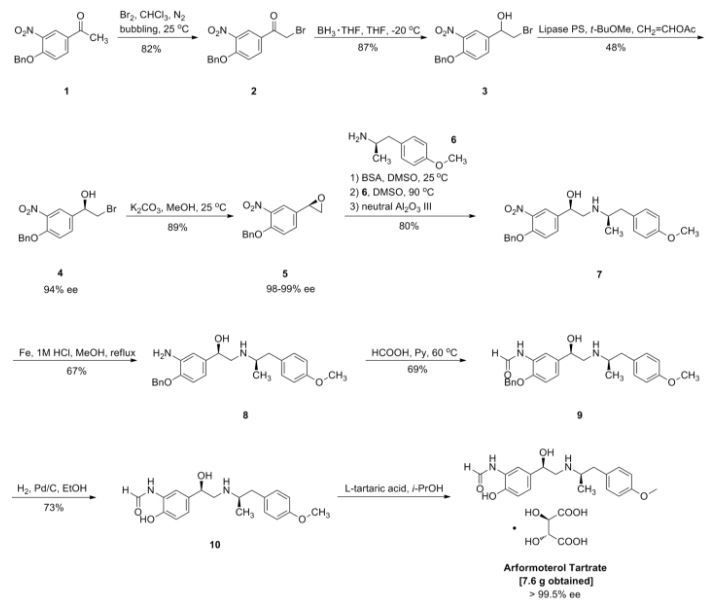
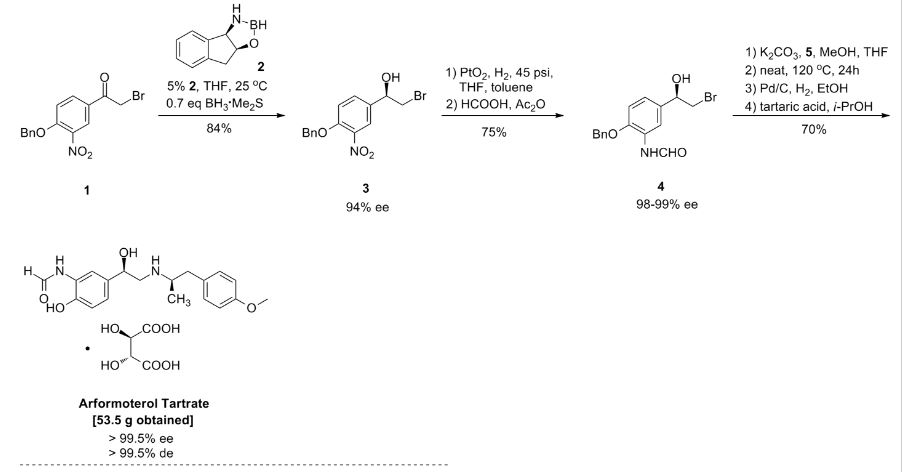
Large-Scale Synthesis of Enantio- and Diastereomerically Pure (R,R)-Formoterol†
Process Research and Development, Sepracor Inc., 111 Locke Drive, Marlborough, Massachusetts 01752
Org. Proc. Res. Dev., 1998, 2 (2), pp 96–99
DOI: 10.1021/op970116o
Abstract
(R,R)-Formoterol (1) is a long-acting, very potent β2-agonist, which is used as a bronchodilator in the therapy of asthma and chronic bronchitis. Highly convergent synthesis of enantio- and diastereomerically pure (R,R)-formoterol fumarate is achieved by a chromatography-free process with an overall yield of 44%. Asymmetric catalytic reduction of bromoketone 4 using as catalyst oxazaborolidine derived from (1R, 2S)-1-amino-2-indanol and resolution of chiral amine 3 are the origins of chirality in this process. Further enrichment of enantio- and diastereomeric purity is accomplished by crystallizations of the isolated intermediates throughout the process to give (R,R)-formoterol (1) as the pure stereoisomer (ee, de >99.5%).
(R,R)-formoterol fumarate (53.5 g, 70%) as white crystals: mp = 139 °C dec; [α]20D = −45.5 (c = 1, H2O); ee, de > 99.5%; 1H NMR (300 MHz, DMSO-d6) δ (ppm) 9.64 (s), 9.35 (d), 8.55 (d), 8.29 (s), 8.15 (s), 7.14 (d, 2 H), 7.0 (m), 6.95 (d, 2 H), 6.51 (s, 1 H), 4.82 (m, 1 H), 3.72 (s, 3 H), 3.35 (m, 1 H), 3.10 (m, 3 H), 2.58 (m, 1 H), 2.50 (br s, 2 H), 1.06 (d, 3 H).
Anal. Calcd for C42H52N4O12: C, 62.67; H, 6.51; N, 6.96. Found: C, 62.34; H, 6.57; N, 6.85.
Scheme

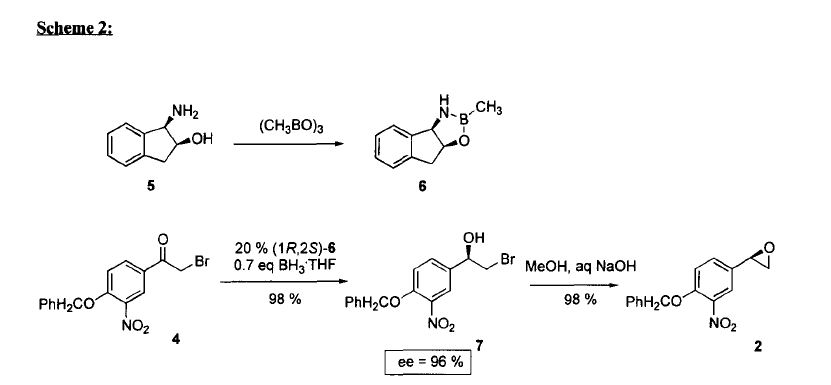
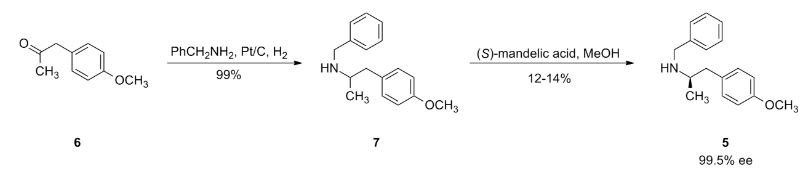
The intermediate N-benzyl-N-[1(R)-methyl-2-(4-methoxyphenyl)ethyl]amine (IV) has been obtained as follows: The reductocondensation of 1-(4-methoxyphenyl)-2-propanone (I) with benzylamine (II) by H2 over Pd/C gives the N-benzyl-N-[1-methyl-2-(4-methoxyphenyl)ethyl]amine (III) as a racemic mixture, which is submitted to optical resolution with L-mandelic acid in methanol to obtain the desired (R)-enantiomer (IV). The reaction of cis-(1R,2S)-1-aminoindan-2-ol (V) with trimethylboroxine in toluene gives the (1R,2S)-oxazaborolidine (VI), which is used as chiral catalyst in the enantioselective reduction of 4-benzyloxy-3-nitrophenacyl bromide (VII) by means of BH3/THF, yielding the chiral bromoethanol derivative (VIII). The reaction of (VIII) with NaOH in aqueous methanol affords the epoxide (IX), which is condensed with the intermediate amine (IV) by heating the mixture at 90 C to provide the adduct (X). The reduction of the nitro group of (X) with H2 over PtO2 gives the corresponding amino derivative (XI), which is acylated with formic acid to afford the formamide compound (XII). Finally, this compound is debenzylated by hydrogenation with H2 over Pd/C in ethanol, providing the target compound.

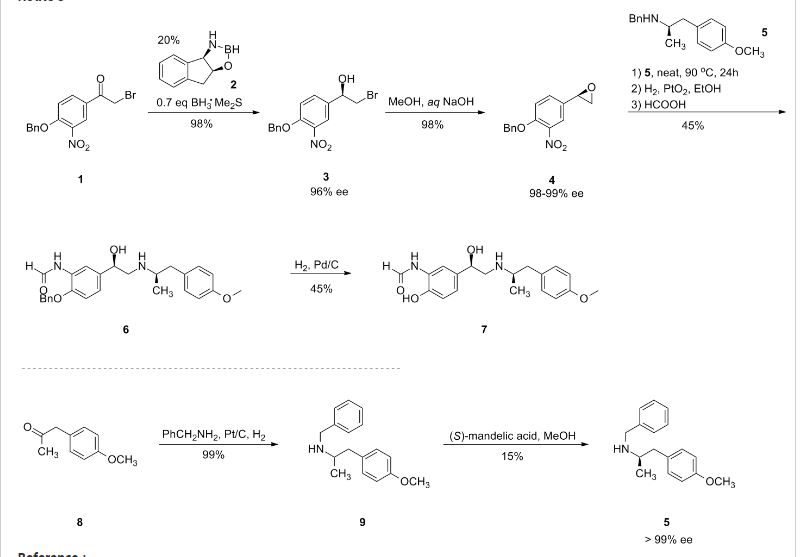
The synthesis of the chiral borolidine catalyst (II) starting from indoline (I), as well as the enantioselective reduction of 4′-(benzyloxy)-3′-nitrophenacyl bromide (III), catalyzed by borolidine (II), and using various borane complexes (borane/dimethylsulfide, borane/THF and borane/diethylaniline), has been studied in order to solve the problems presented in large-scale synthesis. The conclusions of the study are that the complex borane/diethylaniline (DEANB) is the most suitable reagent for large-scale reduction of phenacyl bromide (III) since the chemical hazards and inconsistent reagent quality of the borane/THF and borane/dimethylsulfide complexes disqualified their use in large-scale processes. The best reaction conditions of the reduction with this complex are presented.
PATENT
Formoterol is a long-acting β2-adrenoceptor agonist and has a long duration of action of up to 12 hours. Chemically it is termed as Λ/-[2-hydroxy-5-[1-hydroxy-2-[[2-(4- methoxyphenyl)propan-2-yl]amino]ethyl]phenyl]-formamide. The structure of formoterol is as shown below.
The asterisks indicate that formoterol has two chiral centers in the molecule, each of which can exist in two possible configurations. This gives rise to four diastereomers which have the following configurations: (R,R), (S1S), (S1R) and (R1S).
(R1R) and (S1S) are mirror images of each other and are therefore enantiomers. Similarly (S1R) and (R1S) form other enatiomeric pair.
The commercially-available formoterol is a 50:50 mixture of the (R1R)- and (S1S)- enantiomers. (R,R)-formoterol is an extremely potent full agonist at the β2-adrenoceptor and is responsible for bronchodilation and has anti-inflammatory properties. On the other hand (S,S)-enantiomer, has no bronchodilatory activity and is proinflammatory.
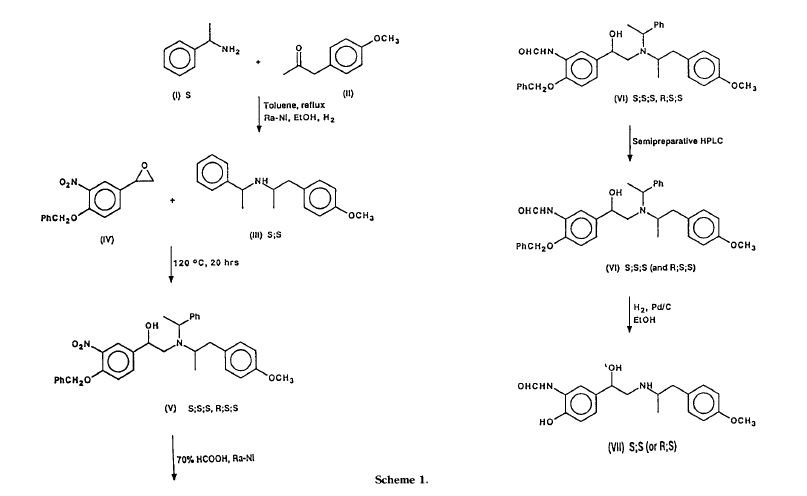
Murase et al. [Chem.Pharm.Bull., .26(4)1123-1129(1978)] synthesized all four isomers of formoterol and examined for β-stimulant activity. In the process, racemic formoterol was subjected to optical resolution with tartaric acid.
In another attempt by Trofast et al. [Chirality, 3:443-450(1991 )], racemic 4-benzyloxy-3- nitrostryrene oxide was coupled with optically pure N-[(R)-1-phenylethyl]-2-(4- methoxyphenyl)-(R)1-methylethylamine to give diastereomeric mixtures of intermediates, which were separated by column chromatography and converted to the optically pure formoterol.
In yet another attempt, racemic formoterol was subjected to separation by using a chiral compound [International publication WO 1995/018094].
WO 98/21175 discloses a process for preparing optically pure formoterol using optically pure intermediates (R)-N-benzyl-2-(4-methoxyphenyl)-1-methylethyl amine and (R)-4- benzyloxy-3-formamidostyrene oxide.
Preparation of optically pure formoterol is also disclosed in IE 000138 and GB2380996.

Example 7
Preparation of Arformoterol
4-benzyloxy-3-formylamino-α-[N-benzyl-N-(1-methyl-2-p- methoxyphenylethyl)aminomethyl]benzyl alcohol (120gms, 0.23M), 10% Pd/C (12 gms) and denatured spirit (0.6 lit) were introduced in an autoclave. The reaction mass was hydrogenated by applying 4 kg hydrogen pressure at 25-300C for 3 hrs. The catalyst was removed by filtration and the, clear filtrate concentrated under reduced pressure below 400C to yield the title compound. (63 gms, 80%).
Example 8
Preparation of Arformoterol Tartrate
Arformoterol base (60 gms, 0.17M), 480 ml IPA , 120 ml toluene and a solution of l_(+)- tartaric acid (25.6 gms, 0.17M) in 60 ml distilled water were stirred at 25-300C for 2 hrs and further at 40°- 45°C for 3 hrs. The reaction mass was cooled to 25-300C and further chilled to 200C for 30 mins. The solid obtained was isolated by filtration to yield the title compound. (60 gms, 70%),
The tartrate salt was dissolved in hot 50% IPA-water (0.3 lit), cooled as before and filtered to provide arformoterol tartrate. (30 gms, 50 % w/w). having enantiomeric purity greater than 99%.

PAPER
Organic Process Research & Development 2000, 4, 567-570
Modulation of Catalyst Reactivity for the Chemoselective Hydrogenation of a Functionalized Nitroarene: Preparation of a Key Intermediate in the Synthesis of (R,R)-Formoterol Tartrate………..
http://pubs.acs.org/doi/abs/10.1021/op000287k
Modulation of Catalyst Reactivity for the Chemoselective Hydrogenation of a Functionalized Nitroarene: Preparation of a Key Intermediate in the Synthesis of (R,R)-Formoterol Tartrate
Chemical Research and Development, Sepracor Inc., 111 Locke Drive, Marlborough, Massachusetts 01752, U.S.A.
Org. Proc. Res. Dev., 2000, 4 (6), pp 567–570
DOI: 10.1021/op000287k
In the synthesis of the β2-adrenoceptor agonist (R,R)-formterol, a key step in the synthesis was the development of a highly chemoselective reduction of (1R)-2-bromo-1-[3-nitro-4-(phenylmethoxy)phenyl]ethan-1-ol to give (1R)-1-[3-amino-4-(phenylmethoxy)phenyl]-2-bromoethan-1-ol. The aniline product was isolated as the corresponding formamide. The reaction required reduction of the nitro moiety in the presence of a phenyl benzyl ether, a secondary benzylic hydroxyl group, and a primary bromide, and with no racemization at the stereogenic carbinol carbon atom. The development of a synthetic methodology using heterogeneous catalytic hydrogenation to perform the required reduction was successful when a sulfur-based poison was added. The chemistry of sulfur-based poisons to temper the reacitivty of catalyst was studied in depth. The data show that the type of hydrogenation catalyst, the oxidation state of the poison, and the substituents on the sulfur atom had a dramatic effect on the chemoselectivity of the reaction. Dimethyl sulfide was the poison of choice, possessing all of the required characteristics for providing a highly chemoselective and high yielding reaction. The practicality and robustness of the process was demonstrated by preparing the final formamide product with high chemoselectivity, chemical yield, and product purity on a multi-kilogram scale.
PAPER
Tetrahedron: Asymmetry 11 (2000) 2705±2717
An ecient enantioselective synthesis of (R,R)-formoterol, a potent bronchodilator, using lipases
Francisco Campos, M. Pilar Bosch and Angel Guerrero*
formoterol (R,R)-1 as amorphous solid. Rf: 0.27 (SiO2, AcOEt:MeOH, 1:1).�20D=-41.5 (CHCl3, c 0.53).
IR, �: 3383, 2967, 2923, 1674, 1668, 1610, 1514, 1442, 1247, 1033,815 cm^1.
1H NMR (300 MHz, CDCl3), �: 8.11 (b, 1H), 7.46 (b, 1H), 6.99 (d, J=8.4 Hz, 2H), 6.9±6.7 (c, 4H), 4.46 (m, 1H), 4.34 (b, 3H interchangeable), 3.74 (s, 3H), 2.90±2.45 (c, 5H), 1.02 (d,J=5.7 Hz, 3H) ppm.
13C NMR (75 MHz, CDCl3), �: 160.2, 158.3, 147.7, 133.4, 130.6, 130.2 (2C),125.7, 123.7, 119.5, 117.8, 114.0 (2C), 71.3, 55.3, 54.7, 53.6, 42.0, 19.4 ppm.
CI (positive, LC-MS)(m/z, %) 435 (M+1, 100).
The tartrate salt was prepared by dissolving 13.8 mg (0.04 mmol) of(R,R)-1 and 6.0 mg (0.04 mmol) of (l)-(+)-tartaric acid in 150 mL of 85% aqueous isopropanol.
The solution was left standing overnight and the resulting crystalline solid (7.6 mg) puri®ed on areverse-phase column (1 g, Isolute SPE C18) using mixtures of MeOH±H2O as eluent. The solventwas removed under vacuum and the aqueous solution lyophilized (^35�C, 0.6 bar) overnight. The(l)-(+)-tartrate salt of (R,R)-1 showed an �20D=-29.4 (H2O, c 0.61) (>99% ee based on the
reported value 34). 34=Hett, R.; Senanayake, C. H.; Wald, S. A. Tetrahedron Lett. 1998, 39, 1705.
PAPER
Diethylanilineborane: A Practical, Safe, and Consistent-Quality Borane Source for the Large-Scale Enantioselective Reduction of a Ketone Intermediate in the Synthesis of (R,R)-Formoterol
Chemical Research and Development, Sepracor Incorporated, 111 Locke Drive, Marlborough, Massachusetts 01752, U.S.A.
Org. Proc. Res. Dev., 2002, 6 (2), pp 146–148
DOI: 10.1021/op015504b
Abstract
The development of a process for the use of N,N-diethylaniline−borane (DEANB) as a borane source for the enantioselective preparation of a key intermediate in the synthesis of (R,R)-formoterol l-tartrate, bromohydrin 2, from ketone 3 on kilogram scale is described. DEANB was found to be a more practical, safer, and higher-quality reagent when compared to other more conventional borane sources: borane−THF and borane−DMS.
PAPER
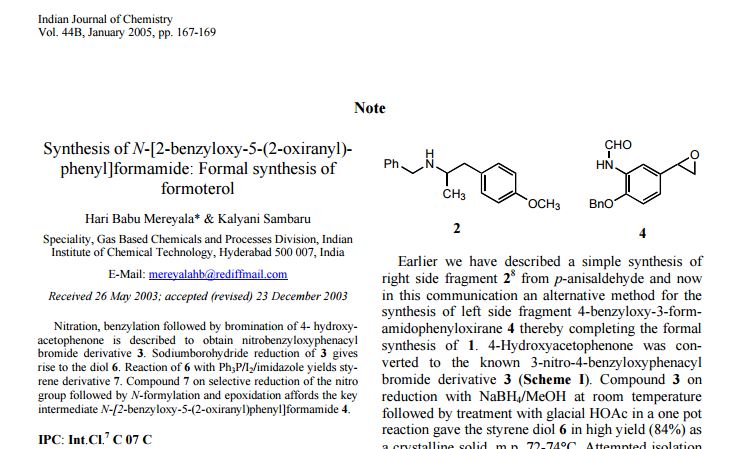
http://nopr.niscair.res.in/bitstream/123456789/8917/1/IJCB%2044B(1)%20167-169.pdf
PAPER
http://www.bioorg.org/down/Hetetorcycles_07_2243.pdf?ckattempt=1


PAPER
Arformoterol: (R,R)-eformoterol, (R,R)-formoterol, arformoterol tartrate, eformoterol-sepracor, formoterol-sepracor, R,R-eformoterol, R,R-formoterol.
Abstract
Sepracor in the US is developing arformoterol [R,R-formoterol], a single isomer form of the beta(2)-adrenoceptor agonist formoterol [eformoterol]. This isomer contains two chiral centres and is being developed as an inhaled preparation for the treatment of respiratory disorders. Sepracor believes that arformoterol has the potential to be a once-daily therapy with a rapid onset of action and a duration of effect exceeding 12 hours. In 1995, Sepracor acquired New England Pharmaceuticals, a manufacturer of metered-dose and dry powder inhalers, for the purpose of preparing formulations of levosalbutamol and arformoterol. Phase II dose-ranging clinical studies of arformoterol as a longer-acting, complementary bronchodilator were completed successfully in the fourth quarter of 2000. Phase III trials of arformoterol began in September 2001. The indications for the drug appeared to be asthma and chronic obstructive pulmonary disease (COPD). However, an update of the pharmaceutical product information on the Sepracor website in September 2003 listed COPD maintenance therapy as the only indication for arformoterol. In October 2002, Sepracor stated that two pivotal phase III studies were ongoing in 1600 patients. Sepracor estimates that its NDA submission for arformoterol, which is projected for the first half of 2004, will include approximately 3000 adult subjects. Sepracor stated in July 2003 that it had completed more than 100 preclinical studies and initiated or completed 15 clinical studies for arformoterol inhalation solution for the treatment of bronchospasm in patients with COPD. In addition, Sepracor stated that the two pivotal phase III studies in 1600 patients were still progressing. In 1995, European patents were granted to Sepracor for the use of arformoterol in the treatment of asthma, and the US patent application was pending.
CLIP
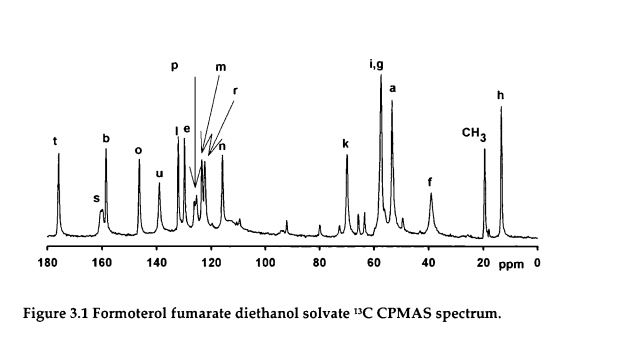
PAPER
doi:10.1016/j.cclet.2008.01.012
http://www.sciencedirect.com/science/article/pii/S1001841708000132
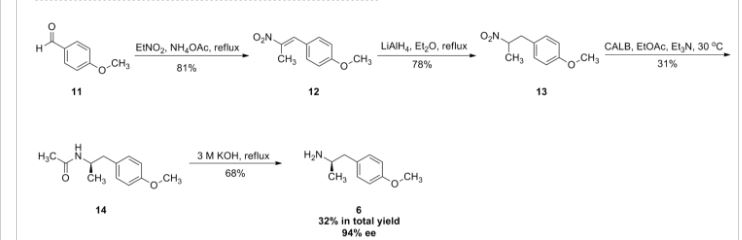
New method in synthesizing an optical active intermediate for (R,R)-formoterol
- Key Laboratory of Drug Targeting Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu 610041, China\
Abstract
(R)-1-(4-Methoxyphenyl)propan-2-amine 2a, an optical active intermediate for (R,R)-formoterol, was synthesized from d-alanine in 65% overall yield by using a simple route, which contained protecting amino group, cyclization, coupling with Grignard reagent, reduction and deprotection.
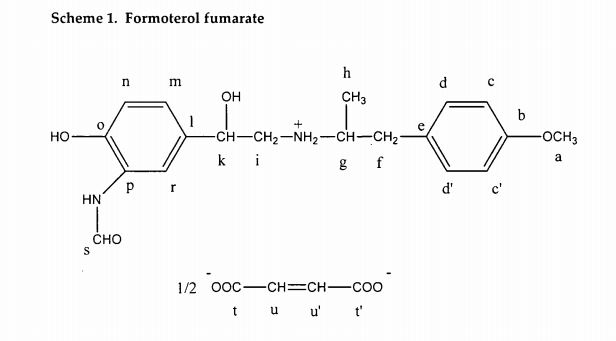
IR spectra of (a) (R,R)-formoterol tartrate/form A, (b) (R,R)-formoterol tartrate/form B, (c) (R,R)-formoterol tartrate/form C.
References
| Muller, P., et al.: Arzneimittel-Forsch., 33, 1685 (1983); Wallmark, B., et al.: Biochim. Biophys. Acta., 778, 549 (1984); Morii, M., et al.: J. Biol. chem., 268, 21553 (1993); Ritter, M., et al.: Br. J. Pharmacol., 124, 627 (1998); Stenhoff, H., et al.: J. Chromatogr., 734, 191 (1999), Johnson, D.A., et al.: Expert Opin. Pharmacother., 4, 253 (2003); Bouyssou, T., et al.: Bio. Med. Chem. Lett. 20, 1410, (2010); |
External links
| EP0390762A1 * |
23 Mar 1990 |
3 Oct 1990 |
Aktiebolaget Draco |
New bronchospasmolytic compounds and process for their preparation |
| EP0938467A1 |
7 Nov 1997 |
1 Sep 1999 |
Sepracor, Inc. |
Process for the preparation of optically pure isomers of formoterol |
| EP1082293A2 |
20 May 1999 |
14 Mar 2001 |
Sepracor Inc. |
Formoterol polymorphs |
| WO2009147383A1 |
2 Jun 2009 |
10 Dec 2009 |
Cipla Limited |
Process for the synthesis of arformoterol |
| Reference |
| 1 |
* |
HETT R ET AL: “Enantio- and Diastereoselective Synthesis of all Four Stereoisomers of Formoterol” TETRAHEDRON LETTERS, ELSEVIER, AMSTERDAM, NL LNKD- DOI:10.1016/S0040-4039(97)00088-9, vol. 38, no. 7, 17 February 1997 (1997-02-17), pages 1125-1128, XP004034214 ISSN: 0040-4039 |
| 2 |
* |
LING HUANG ET AL.: “The Asymmetric Synthesis of (R,R)-Formoterol via Transfer Hydrogenation with Polyethylene Glycol Bound Rh Catalyst in PEG2000 and Water” CHIRALITY, vol. 22, 30 April 2009 (2009-04-30), pages 206-211, XP002592699 |
| 3 |
|
MURASE ET AL. CHEM. PHARM. BULL. vol. 26, no. 4, 1978, pages 1123 – 1129 |
| 4 |
|
TROFAST ET AL. CHIRALITY vol. 1, 1991, page 443 |
| 5 |
* |
TROFAST J ET AL: “STERIC ASPECTS OF AGONISM AND ANTAGONISM AT BETA-ADRENICEPTORS: SYNTHESIS OF AND PHARMACOLOGICAL EXPERIMENTS WITH THE ENANTIOMERS OF FORMOTEROL AND THEIR DIASTEREOMERS” CHIRALITY, WILEY-LISS, NEW YORK, US LNKD- DOI:10.1002/CHIR.530030606, vol. 3, no. 6, 1 January 1991 (1991-01-01) , pages 443-450, XP002057060 ISSN: 0899-0042 |
| 6 |
|
WILKINSON, H.S ET AL. ORGANIC PROCESS RESEARCH AND DEVELOPMENT vol. 6, 2002, pages 146 – 148 |
https://core.ac.uk/download/pdf/6115604.pdf
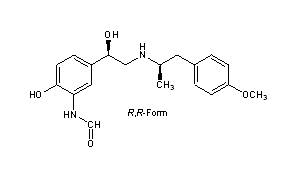
Formoterol
CAS Registry Number: 73573-87-2
CAS Name: rel–N-[2-Hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formamide
Additional Names: 3-formylamino-4-hydroxy-a-[N-[1-methyl-2-(p-methoxyphenyl)ethyl]aminomethyl]benzyl alcohol; (±)-2¢-hydroxy-5¢-[(RS)-1-hydroxy-2-[[(RS)-p-methoxy-a-methylphenethyl]amino]ethyl]formanilide
Molecular Formula: C19H24N2O4
Molecular Weight: 344.40
Percent Composition: C 66.26%, H 7.02%, N 8.13%, O 18.58%
Literature References: Selective b2-adrenergic receptor agonist. Mixture of R,R (-) and S,S (+) enantiomers. Prepn: M. Murakamiet al., DE 2305092; eidem, US 3994974 (1973, 1976 both to Yamanouchi); K. Murase et al., Chem. Pharm. Bull. 25, 1368 (1977). Absolute configuration and activity of isomers: eidem, ibid. 26, 1123 (1978). Toxicity studies: T. Yoshida et al., Pharmacometrics26, 811 (1983). HPLC determn in plasma: J. Campestrini et al., J. Chromatogr. B 704, 221 (1997). Review of pharmacology: G. P. Anderson, Life Sci. 52, 2145-2160 (1993); and clinical efficacy: R. A. Bartow, R. N. Brogden, Drugs 55, 303-322 (1998).
Derivative Type: Fumarate dihydrate
CAS Registry Number: 43229-80-7
Manufacturers’ Codes: BD-40A
Trademarks: Atock (Yamanouchi); Foradil (Novartis); Oxeze (AstraZeneca)
Molecular Formula: (C19H24N2O4)2.C4H4O4.2H2O
Molecular Weight: 840.91
Percent Composition: C 59.99%, H 6.71%, N 6.66%, O 26.64%
Properties: Crystals from 95% isopropyl alcohol, mp 138-140°. pKa1 7.9; pKa2 9.2. Log P (octanol/water): 0.4 (pH 7.4). Freely sol in glacial acetic acid; sol in methanol; sparingly sol in ethanol, isopropanol; slightly sol in water. Practically insol in acetone, ethyl acetate, diethyl ether. LD50 in male, female, rats, mice (mg/kg): 3130, 5580, 6700, 8310 orally; 98, 100, 72, 71 i.v.; 1000, 1100, 640, 670 s.c.; 170, 210, 240, 210 i.p. (Yoshida).
Melting point: mp 138-140°
pKa: pKa1 7.9; pKa2 9.2
Log P: Log P (octanol/water): 0.4 (pH 7.4)
Toxicity data: LD50 in male, female, rats, mice (mg/kg): 3130, 5580, 6700, 8310 orally; 98, 100, 72, 71 i.v.; 1000, 1100, 640, 670 s.c.; 170, 210, 240, 210 i.p. (Yoshida)
Derivative Type: R,R-Form
CAS Registry Number: 67346-49-0
Additional Names: Arformoterol
Derivative Type: R,R-Form L-tartrate
CAS Registry Number: 200815-49-2
Additional Names: Arformoterol tartrate
Molecular Formula: C19H24N2O4.C4H6O6
Molecular Weight: 494.49
Percent Composition: C 55.86%, H 6.12%, N 5.67%, O 32.36%
Literature References: Prepn: Y. Gao et al., WO 9821175; eidem, US 6040344 (1998, 2000 both to Sepracor). Pharmacology: D. A. Handley et al., Pulm. Pharmacol. Ther. 15, 135 (2002).
Properties: Off-white powder, mp 184°.
Melting point: mp 184°
Therap-Cat: Antiasthmatic.
Keywords: ?Adrenergic Agonist; Bronchodilator; Ephedrine Derivatives.
//////Arformoterol, (R,R)-Formoterol, (R,R)-Formoterol-L-(+)-tartrate, 200815-49-2, Arformoterol tartrate , Brovana, UNII:5P8VJ2I235, Sepracor, Asthma Therapy, Bronchodilators, Chronic Obstructive Pulmonary Diseases, COPD , RESPIRATORY DRUGS, beta2-Adrenoceptor Agonists, Phase III, 2007, Sunovion
COC1=CC=C(C[C@@H](C)NC[C@H](O)C2=CC(NC=O)=C(O)C=C2)C=C1














 3D STRUCTURE
3D STRUCTURE Arformoterol ball-and-stick model
Arformoterol ball-and-stick model