As a GUEST BLOGGER, myself Dr Pravin Patil, presenting my paper as below
A New Combination of Cyclohexylhydrazine and IBX for Oxidative Generation of Cyclohexyl Free Radical and Related Synthesis of Parvaquone
Pravin C Patil*a and Krishnacharya G Akamanchi
Department of Pharmaceutical Sciences and Technology, Institute of Chemical Technology, Matunga, Mumbai-400 019.
aPresent address: Department of Chemistry, University of Louisville, Louisville, KY, USA.
*Corresponding Author: Email-pravinchem@gmail.com
Tetrahedron Letters 2017, 58 (19), 1883-1886 (Recently published)
[Link: http://www.sciencedirect.com/science/article/pii/S004040391730429X]
Graphical Abstract:
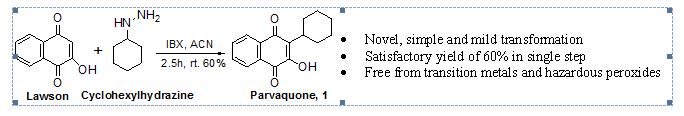
Abstract: The present paper demonstrate a single-step and straightforward synthesis of parvaquone through intermediacy of cyclohexyl radical generated from novel combination of cyclohexylhydrazine and o-iodoxybenzoic acid and subsequently trapped by 2-hydroxy-1,4-naphthoquinone. Formation of cyclohexyl free radical using this new combination was reaffirmed by cyclohexylation of readily available 2-amino-1, 4-naphthoquinone.
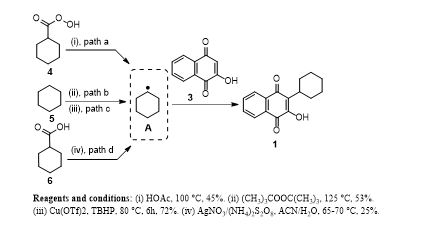
Scheme: Literature methods for synthesis of parvaquone
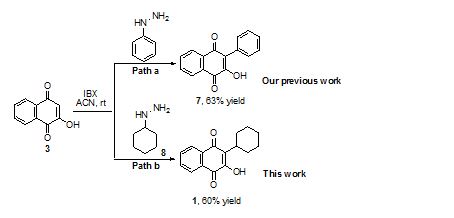
Scheme: IBX mediated oxidative arylation towards synthesis of 1 (Parvaquone)
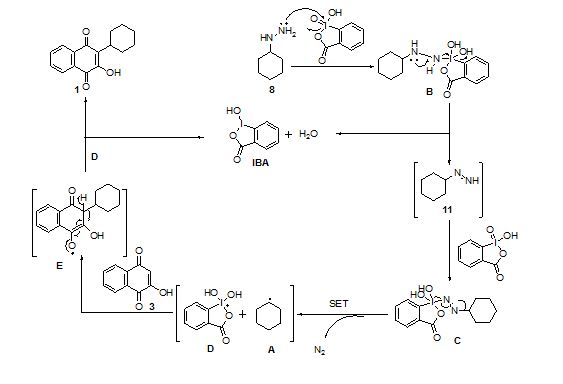
Scheme : Cyclohexyl radical mediated postulated mechanism for formation of Parvaquone, 1
Synthesis of 2-cyclohexyl-3-hydroxy-1,4-naphthoquinone (parvaquone) (1): To a solution of 3 (1.0 g, 5.74 mmol) in acetonitrile (20 mL) was added IBX (3.80 g, 13.6 mmol) in one lot and stirred for 5 min at room temperature. To this was added dropwise a solution of 8 (0.78 g, 6.8 mmol) dissolved in 10 mL of acetonitrile over the course of 20 min. During the addition of 8 exotherm (up to 35 °C) was observed with evolution of nitrogen gas in the form of bubbles. Reaction progress was monitored by TLC (using mobile phase, hexane: ethyl acetate/5:95). After satisfactory TLC, water (20 mL) was added to the reaction mixture and acetonitrile was evaporated using rotary evaporator. To the residue obtained was added dichloromethane (30 mL). Oganic layer was separated and washed with saturated sodium bicarbonate solution followed by saturated solution of sodium sulphite. Separated organic layer was dried over anhydrous sodium sulphate and evaporated to obtain crude 1 which was further purified by column chromatography (mobile phase – hexane: ethyl acetate/5:95) to afford 1 as yellow solid, (0.88 g, 60% yield); mp 136-138 °C (lit.18 135-136°C); FT-IR (KBr): 3585, 3513, 3071, 2926, 2853, 1666, 1604, 1590 cm-1;
1H NMR (300 MHz; CDCl3): δ 8.10-8.06 (d, J = 12 Hz, 2H), 7.74-7.67 (d, J = 22 Hz, 2H, 7.45 (s, 1H, OH), 3.11-3.03 (t, J = 16 Hz, 1H), 1.99-1.34 (m, 10H); 13C NMR (75 MHz; CDCl3): δ 184.5, 181.9, 152.8, 135.1, 134.9, 132.7, 129.2, 127.9, 126.9, 125.9, 35.1, 29.2, 26.7, 25.9.
Highlights
- New method of generating cyclohexyl radical by using IBX and cyclohexylhydrazine.
- Parvaquone synthesized in 60% yield using metal, hazardous peroxide free conditions.
- Described method has advantages of single step and mild reaction conditions.
- The mechanism for cyclohexyl radical mediated synthesis of parvaquone is postulated.
please note………

ABOUT GUEST BLOGGER

Dr. Pravin C. Patil
Postdoctoral Research Associate at University of Louisville
see…….http://oneorganichemistoneday.blogspot.in/2017/04/dr-pravin-patil.html
Dr. Pravin C Patil completed his B.Sc. (Chemistry) at ASC College Chopda (Jalgaon, Maharashtra, India) in 2001 and M.Sc. (Organic Chemistry) at SSVPS’S Science College Dhule in North Maharashtra University (Jalgaon, Maharashtra, India) in year 2003. After M.Sc. degree he was accepted for summer internship training program at Bhabha Atomic Research Center (BARC, Mumbai) in the laboratory of Prof. Subrata Chattopadhyay in Bio-organic Division. In 2003, Dr. Pravin joined to API Pharmaceutical bulk drug company, RPG Life Science (Navi Mumbai, Maharashtra, India) and worked there for two years. In 2005, he enrolled into Ph.D. (Chemistry) program at Institute of Chemical Technology (ICT), Matunga, Mumbai, aharashtra, under the supervision of Prof. K. G. Akamanchi in the department of Pharmaceutical Sciences and Technology.
After finishing Ph.D. in 2010, he joined to Pune (Maharashtra, India) based pharmaceutical industry, Lupin Research Park (LRP) in the department of process development. After spending two years at Lupin as a Research Scientist, he got an opportunity in June 2012 to pursue Postdoctoral studies at Hope College, Holland, MI, USA under the supervision of Prof. Moses Lee. During year 2012-13 he worked on total synthesis of achiral anticancer molecules Duocarmycin and its analogs. In 2014, he joined to Prof. Frederick Luzzio at the Department for Chemistry, University of Louisville, Louisville, KY, USA to pursue postdoctoral studies on NIH sponsored project “ Structure based design and synthesis of Peptidomimetics targeting P. gingivalis.
During his research experience, he has authored 23 international publications in peer reviewed journals and inventor for 4 patents.
//////////////Parvaquone, guest blogger, pravin patil