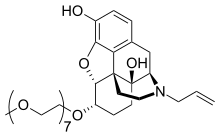
naloxegol
Morphinan-3,14-diol, 4,5-epoxy-6-(3,6,9,12,15,18,21-heptaoxadocos-1-yloxy)-17-(2-
propen-1-yl)-, (5α,6α)-
2. 4,5α-epoxy-6α-[(3,6,9,12,15,18,21-heptaoxadocosan-1-yl)oxy]-17-(prop-2-en-1-
yl)morphinan-3,14-diol
http://www.ama-assn.org/resources/doc/usan/naloxegol.pdf
MOLECULAR FORMULA C34H53NO11
MOLECULAR WEIGHT 651.8
SPONSOR AstraZeneca
CODE DESIGNATION NKTR-118
CAS REGISTRY NUMBER 854601-70-0
The US Food and Drug Administration (FDA) has accepted AstraZeneca’s new drug application (NDA) for naloxegol, an investigational peripherally acting mu-opioid receptor antagonist (PAMORA) for the treatment of opioid-induced constipation (OIC).
read at
FDA accepts AstraZeneca’s new drug application for constipation drug
Naloxegol (INN; NKTR-118), or PEGylated naloxol,[1] is a peripherally–selective opioid antagonist under development by AstraZeneca, licensed from Nektar, for the treatment of opioid-induced constipation.[2]
- Roland Seifert; Thomas Wieland; Raimund Mannhold; Hugo Kubinyi, Gerd Folkers (17 July 2006). G Protein-Coupled Receptors as Drug Targets: Analysis of Activation and Constitutive Activity. John Wiley & Sons. p. 227. ISBN 978-3-527-60695-5. Retrieved 14 May 2012.
- “Nektar | R&D Pipeline | Products in Development | CNS/Pain | Oral Naloxegol (NKTR-118) and Oral NKTR-119”. Retrieved 2012-05-14
NALOXEGOL OXALATE

credit kegg
NALOXEGOL OXALATE
http://www.ama-assn.org/resources/doc/usan/naloxegol-oxalate.pdf
Morphinan-3,14-diol, 4,5-epoxy-6-(3,6,9,12,15,18,21-heptaoxadocos-1-yloxy)-
17-(2-propen-1-yl)-, (5α,6α)-, ethanedioate (1:1)
2. 4,5α-epoxy-6α-[(3,6,7,12,15,18,21-heptaoxadocosyl)oxy]-17-(prop-2-
enyl)morphinan-3,14-diol hydrogen ethanedioate
MOLECULAR FORMULA C34H53NO11 . C2H2O4
MOLECULAR WEIGHT 741.8
SPONSOR AstraZeneca
CODE DESIGNATIONS NKTR-118 oxalate, AZ13337019 oxalate
CAS REGISTRY NUMBER 1354744-91-4
About Opioid-Induced Constipation
Opioids are commonly prescribed to patients experiencing chronic pain, which can provide relief from serious medical conditions including osteoarthritis, cancer, and chronic back pain.1 There are about 250 million opioid prescriptions written annually in the US alone to treat these conditions.2 Patients taking opioids to treat chronic pain commonly experience a side effect known as opioid-induced constipation, which may include infrequent bowel movements and difficulty passing stools or emptying bowels.1,3 Clinically, OIC is the most prevalent side effect of opioid therapy.4 For those patients who take opiates for long term pain management, approximately 40-50 percent commonly experience OIC.5 Only about 40-50 percent of those patients experience effective relief from current treatment options.6,7
About Naloxegol (NKTR-118)
Naloxegol (NKTR-118) is an investigational drug candidate in Phase 3 studies being developed as a once-daily oral tablet for the treatment of opioid-induced constipation. Naloxegol (NKTR-118) was designed using Nektar’s proprietary small molecule polymer conjugate technology. Results of the Phase 2 study of naloxegol (NKTR-118) were presented in October 2009 at the American College of Gastroenterology Annual Clinical Meeting and the American Academy of Pain Management. NKTR-119 is an early stage drug development program that is intended to combine oral naloxegol (NKTR-118) with selected opioids, with the goal of treating pain without the side effect of constipation traditionally associated with opioid therapy.
Nektar and AstraZeneca have a global agreement for both naloxegol (NKTR-118) and NKTR-119. Under the agreement, AstraZeneca has responsibility for the development, global manufacturing and marketing of both naloxegol (NKTR-118) and NKTR-119. For naloxegol (NKTR-118), Nektar is eligible to receive up to $235 million in aggregate payments upon the achievement of certain regulatory milestones, as well as additional tiered sales milestone payments of up to $375 million if the product achieves considerable levels of commercial success. Nektar will also be eligible to receive significant double-digit royalty payments on net sales of naloxegol (NKTR-118) worldwide. For NKTR-119, Nektar would receive development milestone payments as well as tiered sales milestone payments. Nektar will also receive significant double-digit royalty payments on NKTR-119 net sales worldwide.
| The AstraZeneca Phase 3 KODIAC Program for Naloxegol (NKTR-118) The KODIAC Program consists of two randomized, placebo controlled Phase III efficacy studies and an open-label, randomized, placebo-controlled long term safety study. The two efficacy studies are identical with 12-week treatment periods. These studies are intended to evaluate the efficacy, safety and tolerability of an AstraZeneca investigational drug in patients with OIC. KODIAC is part of the KODIAC program of studies looking to determine whether naloxegol (NKTR-118) is safe and effective for the treatment of constipation seen as a side effect in people taking prescription opioid pain medications. AstraZeneca plans the first regulatory filings based on the program in 2013. |
References
http://newdrugapprovals.wordpress.com/2013/09/28/ema-accepts-astrazenecas-naloxegol-application/











