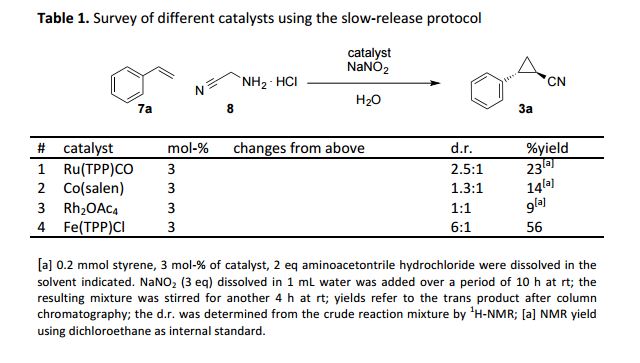
Towards nitrile-substituted cyclopropanes – a slow-release protocol for safe and scalable applications of diazo acetonitrile
Green Chem., 2017, Advance Article
DOI: 10.1039/C7GC00602K, Communication
Katharina J. Hock, Robin Spitzner, Rene M. Koenigs
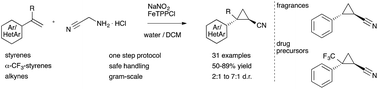
Applications of diazo acetonitrile in cyclopropa(e)nation reactions are realized in a slow-release protocol with bench-stable reagents. Cyclopropyl nitriles are obtained in one step in good diastereoselectivity on a gram-scale providing an efficient entry into this class of fragrances and drug-like molecules.
Applications of diazo acetonitrile in cyclopropa(e)nation reactions are realized in a slow-release protocol with bench-stable reagents. Cyclopropyl nitriles are obtained in one step in good diastereoselectivity on a gram-scale providing an efficient entry into this class of fragrances and drug-like molecules.
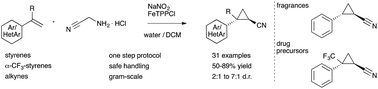
trans-2-phenylcyclopropane-1-carbonitrile
colorless solid (46 mg, 81%);
m.p. = 29°C;
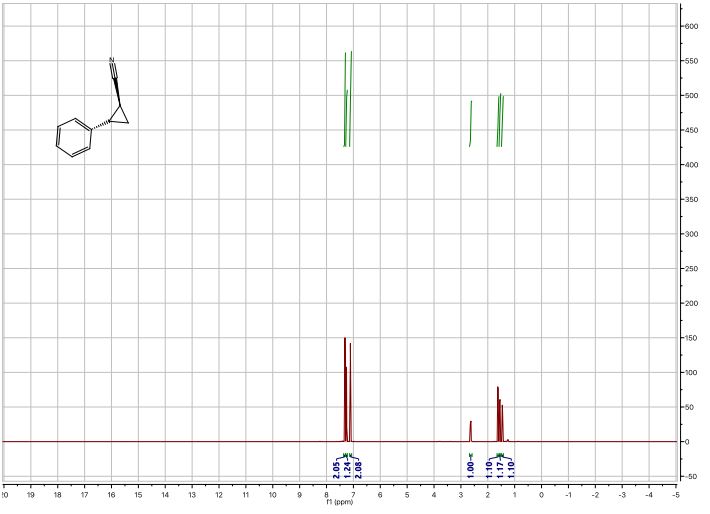
1 H-NMR (600 MHz, CDCl3): δ = 7.34 – 7.30 (m, 2H), 7.28 – 7.24 (m, 1H), 7.12 – 7.08 (m, 2H), 2.63 (ddd, J = 9.2, 6.7, 4.7 Hz, 1H), 1.62 (dt, J = 9.2, 5.4 Hz, 1H), 1.55 (ddd, J = 8.7, 5.5, 4.8 Hz, 1H), 1.45 (ddd, J = 8.8, 6.7, 5.3 Hz, 1H);
13C-NMR (151 MHz, CDCl3): δ = 137.55, 128.76, 127.41, 126.31, 121.05, 24.90, 15.24, 6.63;
HRMS (ESI): m/z calc. for [C10H9NNa]: 166.06272, found 166.06276;
IR (KBr): νmax/cm-1 = 3044, 2235, 2098, 1761, 1600, 1461, 1220, 1051, 920, 705.
The analytical data is in correspondence with the literature [2]
[2] M. Gao, N. N. Patwardhan, P. R. Carlier, J. Am. Chem. Soc., 2013, 135 (38), 14390–14400
Towards nitrile-substituted cyclopropanes – a slow-release protocol for safe and scalable applications of diazo acetonitrile
Abstract
Diazo acetonitrile has long been neglected despite its high value in organic synthesis due to a high risk of explosions. Herein, we report our efforts towards the transient and safe generation of this diazo compound, its applications in iron catalyzed cyclopropanation and cyclopropenation reactions and the gram-scale synthesis of cyclopropyl nitriles.
//////////