A methodology for chemical routes development and evaluation on the basis of data-mining is presented. A section of the Reaxys database was converted into a network, which was used to plan hypothetical synthesis routes to convert a bio-waste feedstock, limonene, to a bulk intermediate, benzoic acid. The route evaluation considered process conditions and used multiple indicators, including exergy, E-factor, solvent score, reaction reliability and route redox efficiency, in a multi-criteria environmental sustainability evaluation. The proposed methodology is the first route evaluation based on data mining, explicitly using reaction conditions, and is amenable to full automation.
In the field of process and synthetic chemistry ‘clean synthesis’ has become one of the standard criteria for good, commercially viable synthesis routes. As a result synthetic and process chemists must be equipped with adequate methodologies for quantification of ‘cleanness’ or ‘greenness’ of alternative routes at the early phases of the development cycle. These new criteria, and the traditional criteria of cost, security of supply, health and safety (H&S), and risk, provide a balanced picture of sustainability of a future technology. Thus, there are two separate aspects to process chemistry: developing the chemistry and the process, and evaluating the overall process, which must occur in parallel. Evaluation of the proposed routes requires data. As data science rapidly evolves, chemistry will inevitably use more of the new tools of data mining and data analysis to automate the routine tasks, such as evaluation of process metrics. In this paper we show some initial results in automation of process evaluation based on deep data mining of process chemistry and multi-criteria decision making.
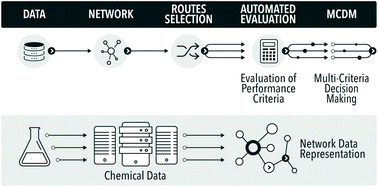
The evaluation of greenness is a mature field, with a large number of published and standardised approaches, of which many are adopted by industry. 1 However, all published methods are highly case-specific and rather labour-intensive. In the field of synthetic routes development one of the most exciting new areas is the potential for automation of synthesis planning using data mining.2 What has never been attempted before is to automate route generation and evaluation in a coherent methodology, which would aid process development at the early, data-lean, stages. For this we show how to automatically generate process options using a network representation of a section of Reaxys database,3 followed by their screening using multi-criteria decision making, see Fig. 1. As the methods mature and become commercially available, such integration and automation will produce significant savings of time, and would deliver a far more detailed view of the competing synthesis route options than is generally possible at the early stages of design.
To date, obtaining the data, assembling the network and finding potential synthesis routes can already be carried out in a fully automated fashion. Due to issues around data availability the connection to the analysis of the routes still has to be initiated manually, involving a data curation step. The subsequent analysis and multi-criteria decision making have been largely automated in this study. To our knowledge this is the first example of the analysis of synthesis routes generated from the network representation of Reaxys obtained through datamining, using reaction conditions and process data.
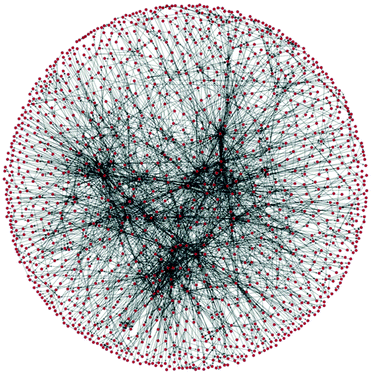
| Fig. 2 A section of a network of organic chemistry. Dots are species and arrows represent reactions. |
- D. J. C. Constable, C. Jimenez-Gonzalez and A. Lapkin, in Green Chemistry Metrics, John Wiley & Sons, Ltd, Chichester, UK, 2009, pp. 228–247
- S. Szymkuć, E. P. Gajewska, T. Klucznik, K. Molga, P. Dittwald, M. Startek, M. Bajczyk and B. A. Grzybowski, Angew. Chem., Int. Ed., 2016, 55, 5904–5937
- Reed Elsevier Properties SA, Login – Reaxys Login Page [Internet], 2014 [accessed 2014 Jun 8]. Available from: https://www.reaxys.com/. Reaxys is a trademark, copyright owned by Relex Intellectual properties SA and used under licence.
Towards automation of chemical process route selection based on data mining
E-mail: aal35@cam.ac.uk
DOI: 10.1039/C6GC02482C, http://pubs.rsc.org/en/Content/ArticleLanding/2017/GC/C6GC02482C?utm_medium=email&utm_campaign=pub-GC-vol-19-issue-1&utm_source=toc-alert#!divAbstract
Professor Alexei Lapkin FRSC
Professor of Sustainable Reaction Engineering
Fellow of Wolfson College
Catalytic Reaction Engineering
Sustainable Chemical Technologies
Office Phone: 330141


Biography:
MChem in Biochemistry, Novosibirsk State University, 1994
PhD in Chemical Engineering, University of Bath, 2000
Boreskov Institute of Catalysis, Novosibirsk, Russia (1994-1997)
University of Bath, Department of Chemical Engineering, Research Officer (1997-2000)
University of Bath, Department of Chemical Engineering, Lecturer-SL-Reader (2000-2009)
University of Warwick, School of Engineering, Professor of Engineering (2009-2013)
Research Interests
Our group is developing cleaner manufacturing processes within chemical and chemistry using industries. We are mainly focusing on liquid- and multi-phase catalytic and biochemical processes. Within the group we have pursued projects on developing functional materials for catalysts, adsorbents and reactors, design of multi-functional intensive reactors, modelling of reaction kinetics and integrated processes, linking reaction kinetics with computational fluid dynamics (CFD) and linking process modelling with life cycle assessment (LCA), integration of reactions and separation.
Public funding:
The group is currently involved in an EU project ‘RECOBA’ (http://www.spire2030.eu/recoba/), in which our group collaborates with Materials and Electronic Engineering at Cambridge to work on innovative measurement techniques for monitoring processes under reaction conditions.
We are involved in the EPSRC project on developing novel routes to platform and functional molecules from waste terpenes, led by University of Bath.
We are involved in “Dial a Molecule 2” network funded by EPSRC.
Keywords
|
Key Publications
J. Zakrzhewski, A.P. Smalley, M. Kabeshov, A. Lapkin, M. Gaunt, Continuous flow synthesis and derivatization of aziridines via palladium-catalyzed C(sp3)-H activation, Angew. Chem. Int. Ed., 55 (2016) 8878-8883.
P. Yaseneva, P. Hodgson, J. Zakrzewski, S. Falss, R.E. Meadows, A.A. Lapkin, Continuous flow Buchwald-Hartwig amination of a pharmaceutical intermediate, React. Chem. Eng., 1 (2016) 229-238.
P. Yaseneva, D. Plaza, X. Fan, K. Loponov, A. Lapkin, Synthesis of the antimalarial API artemether in a flow reactor, Catal. Today, 239 (2015) 90-96.
N. Peremezhney, E. Hines, A. Lapkin, C. Connaughton, Combining Gaussian processes, mutual information and a generic algorithm for multi-targeted optimisation of expensive-to-evaluate functions, Engineering Optimisation, 46 (2014) 1593-1607.
P. Yaseneva, C.F. Marti, E. Palomares, X. Fan, T. Morgan,P.S. Perez, M. Ronning, F. Huang,T. Yuranova, L. Kiwi-Minsker, S. Derrouiche, A.A. Lapkin, Efficient reduction of bromates using carbon nanofibre supported catalysts: experimental and a comparative life cycle assessment study, Chem. Eng. J., 248 (2014) 230-241
K.N. Loponov, J. Lopes, M. Barlog, E.V. Astrova, A.V. Malkov, A.A. Lapkin, Optimization of a Scalable Photochemical Reactor for Reactions with Singlet Oxygen, Org.Process Res.Dev., 18 (2014) 1443-1454.
X. Fan, V. Sans, P. Yaseneva, D. Plaza, J.M.J. Williams, A.A. Lapkin, Facile Stoichiometric Reductions in Flow: an Example of Artemisinin, Org.Process Res.Dev., 16 (2012) 1039-1042.
M.V. Sotenko, M. Rebros, V.S. Sans, K.N. Loponov, M.G. Davidson, G. Stephens, A.A. Lapkin, Tandem transformation of glycerol to esters, J. Biotechnol., 162 (2012) 390-397.
A.A. Lapkin, A. Voutchkova, P. Anastas, A conceptual framework for description of complexity in intensive chemical processes, Chem. Eng. Processing. Process intensification, 50 (2011) 1027-1034.
Lapkin, A., Peters, M., Greiner, L., Chemat, S., Leonhard, K., Liauw, M. A. and Leitner, W., Screening of new solvents for artemisinin extraction process using ab-initio methodology, Green Chem., 12 (2010) 241-251.
Lapkin, A. A. and Plucinski, P. K., Engineering factors for efficient flow processes in chemical industries, in Chemical reactions and processes under flow conditions, pp. 1- 43, Eds: Luis, S. V. and Garcia-Verdugo, E., Royal Society of Chemistry, Cambridge, 2010.
Iwan, A., Stephenson, H., Ketchie, W. C. and Lapkin, A. A., High temperature sequestration of CO2 using lithium zirconates, Chem. Eng. J., 146 (2009) 249-258.
Constable, D. J. C., Jimenez-Gonzalez, C. and Lapkin A., ‘Process metrics’, in Green chemistry metrics: measuring and monitoring sustainable processes, pp. 228- 247, Eds.: Lapkin, A. and Constable, D. J. C., Wiley-Blackwell, Chichester, 2008.
L.Torrente-Murciano, A.Lapkin, D.V. Bavykin, F.C. Walsh, K. Wilson, Highly selective Pd/titanate nanotubes catalysts for the double bond migration reaction, J. Catal., 245 (2007) 270-276.
A. Lapkin, P. Plucinski, Comparative assessment of technologies for extraction of artemisinin, J. Natural Prod., 69 (2006) 1653-1664.
D.V. Bavykin, A.A. Lapkin, S.T. Kolaczkowski, P.K. Plucinski, Selective oxidation of alcohols in a continuous multifunctional reactor: ruthenium oxide catalysed oxidation of benzyl alcohol, Applied Catal. A: General, 288 (2005) 165-174.

////////automation, chemical process, route selection, data mining