Daratumumab
(Darzalex®)Approved
An anti-CD38 monoclonal antibody used to treat multiple myeloma.
![]()
Research Code HuMax-CD-38; HuMaxCD-38
CAS No.

Daratumumab (HuMax®-CD38)
Daratumumab (Darzalex) is an anti-cancer drug. It binds to CD38.[1] Daratumumab was originally developed by Genmab, but it is now being jointly developed by Genmab along with the Johnson & Johnson subsidiary Janssen Biotech, which acquired worldwide commercialization rights to the drug from Genmab.[2]
Clinical trials
Encouraging preliminary results were reported in June 2012 from a Phase 1/2 clinical trial in relapsed multiple myeloma patients.[3]Updated trial results presented in December 2012 indicate daratumumab is continuing to show promising single-agent anti-myeloma activity.[4] A 2015 study compared monotherapy 8 and 16mg/kg at monthly to weekly intervals.[5]
In November 2015, the U.S. Food and Drug Administration approved daratumumab for treatement of multiple myeloma.[6]
Interference with blood compatibility testing
Daratumumab can also bind to CD38 present on red blood cells and interfere with antibody testing. Patients will show a panreactive antibody panel, including a positive auto-control. Treatment of the antibody panel cells with dithiothreitol (DTT) and repeating testing will effectively negate the binding of daratumumab to CD38 on the RBC surface; however, DTT also inactivates/destroys many antigens on the RBC surface by disrupting disulfide bonds. Fortunately, the only antigen system affected that is associated with common, clinically significant antibodies is Kell, making K-negative RBCs a reasonable alternative when urgent transfusion is indicated.[7]
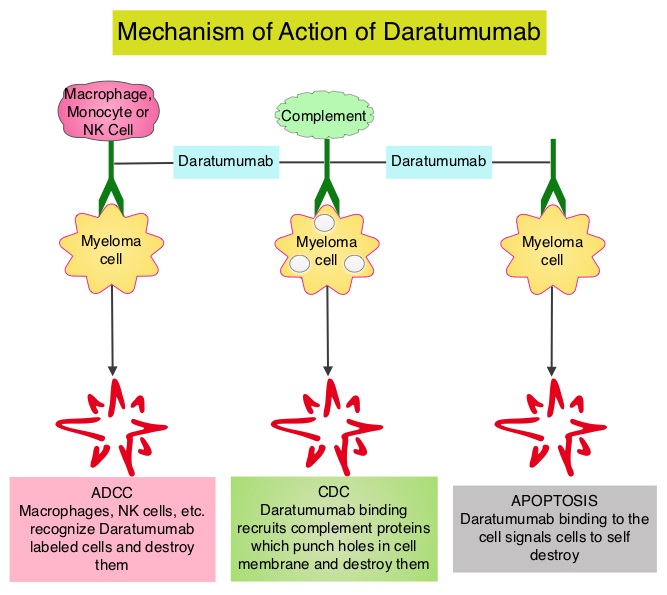
Daratumumab is a human IgG1k monoclonal antibody (mAb) that binds with high affinity to the CD38 molecule, which is highly expressed on the surface of multiple myeloma cells. It is believed to induce rapid tumor cell death through programmed cell death, or apoptosis, and multiple immune-mediated mechanisms, including complement-dependent cytotoxicity, antibody-dependent cellular phagocytosis and antibody-dependent cellular cytotoxicity.
Daratumumab is approved in the United States for the treatment of patients with multiple myeloma who have received at least three prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent, or who are double-refractory to a PI and an immunomodulatory agent.
In May 2013, daratumumab received Fast Track Designation and Breakthrough Therapy Designation from the US FDA for the treatment of patients with multiple myeloma who have received at least three prior lines of therapy including a PI and an immunomodulatory agent or who are double refractory to a PI and an immunomodulatory agent. Breakthrough Therapy Designation is a program intended to expedite the development and review of drugs to treat serious or life-threatening diseases in cases where preliminary clinical evidence shows that the drug may provide substantial improvements over available therapy. Daratumumab has also received Orphan Drug Designation from the US FDA and the EMA for the treatment of multiple myeloma.
Five Phase III clinical studies with daratumumab in relapsed and frontline settings are currently ongoing. Additional studies are ongoing or planned to assess its potential in other malignant and pre-malignant diseases on which CD38 is expressed, such as smoldering myeloma and non-Hodgkin’s lymphoma.
Genmab announced a global license and development agreement for daratumumab with Janssen Biotech, Inc. in August 2012. The agreement became effective in September 2012.
DARZALEX® (daratumumab) Approved by U.S. FDA: First Human Anti-CD38 Monoclonal Antibody Available for the Treatment of Multiple Myeloma
First-in-class immunotherapy approved for multiple myeloma patients who have received three or more prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent or who are double refractory to a PI and immunomodulatory agent
HORSHAM, PA, November 16, 2015 – Janssen Biotech, Inc., a Janssen Pharmaceutical Company of Johnson & Johnson, announced today the U.S. Food and Drug Administration (FDA) has approved DARZALEX® (daratumumab) injection for intravenous infusion for the treatment of patients with multiple myeloma who have received at least three prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent, or who are double-refractory to a PI and an immunomodulatory agent.1 This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. Multiple myeloma is an incurable blood cancer that occurs when malignant plasma cells grow uncontrollably in the bone marrow.2,3 Refractory cancer occurs when a patient’s disease is resistant to treatment or in the case of multiple myeloma, the disease progresses within 60 days of their last therapy.4,5 Relapsed cancer means the disease has returned after a period of initial, partial or complete remission.6
DARZALEX is the first human anti-CD38 monoclonal antibody (mAb) approved anywhere in the world. CD38 is a surface protein that is expressed by most, if not all, multiple myeloma cells.7 DARZALEX is believed to induce tumor cell death through multiple immune-mediated mechanisms of action,8,9 in addition to apoptosis, in which a series of molecular steps in a cell lead to its death.10 Its approval comes just two months after the Biologics License Application (BLA) was accepted for Priority Review by the FDA in September 2015.11 DARZALEX received Breakthrough Therapy Designation from the FDA for this indication in May 2013.12
“Multiple myeloma is a highly complex disease and remains incurable, with almost all patients relapsing or becoming resistant to therapy,” said DARZALEX clinical trial investigator Paul G. Richardson, M.D., Clinical Program Leader and Director of Clinical Research, Jerome Lipper Multiple Myeloma Center, Dana-Farber Cancer Institute. “With DARZALEX, we have a promising new immunotherapy, which has shown pronounced efficacy as a single agent with an acceptable adverse event profile. This is especially important for treating these heavily pre-treated patients in whom all of the major classes of currently available medicines have failed.”
The pivotal open-label Phase 2 MMY2002 (SIRIUS) study showed treatment with single-agent DARZALEX resulted in an overall response rate (ORR) of 29.2 percent (95% CI; 20.8, 38.9) in patients who received a median of five prior lines of therapy, including a PI and an immunomodulatory agent.1
Stringent complete response (sCR) was reported in 2.8 percent of patients, very good partial response (VGPR) was reported in 9.4 percent of patients, and partial response (PR) was reported in 17 percent of patients.1 These efficacy results were based on ORR as determined by the Independent Review Committee assessment using IMWG (International Myeloma Working Group) criteria and the range for median duration of response.
For responders, the median duration of response was 7.4 months (range 1.2-13.1+ months).1 At baseline, 97 percent of patients were refractory to their last line of therapy, 95 percent were refractory to both a PI and an immunomodulatory agent, and 77 percent were refractory to alkylating agents.1 Additional efficacy data from the Phase 1/2 GEN501 monotherapy study – published in The New England Journal of Medicine in August 2015 – also support this approval.1
“The responses we saw in clinical trials that led to today’s approval were striking, especially considering that these patients received a median of five prior lines of therapy,” said MMY2002 investigator Sagar Lonial, M.D., Chief Medical Officer, Winship Cancer Institute of Emory University and Professor and Executive Vice Chair, Department of Hematology and Medical Oncology, Emory University School of Medicine. “It appears the mechanism of action for daratumumab (DARZALEX) may play an important role in its single-agent activity among this group of advanced-stage multiple myeloma patients.”
“Living with multiple myeloma is challenging, both physically and emotionally, especially as the disease progresses and treatment options become more limited,” said Debby Graff, a patient enrolled in a clinical trial at Dana-Farber Cancer Institute. “I am encouraged by emerging treatments for multiple myeloma, and I have a new outlook on my path forward.”
“While there have been considerable improvements over the past decade in the treatment of people living with multiple myeloma, these patients face a long, hard road – especially those whose disease has relapsed or is no longer responding to current therapies,” said Walter M. Capone, President and Chief Executive Officer of the Multiple Myeloma Research Foundation (MMRF). “With the approval of daratumumab, a new antibody option targeting CD38, along with ongoing work to advance the development of novel classes of therapies by both Janssen and MMRF, we are ushering in a new era of myeloma therapy focused on individualized treatment approaches for patients with significant unmet needs.”
“Our focus is developing transformational medicines for people living with hard-to-treat cancers, such as multiple myeloma,” said Peter F. Lebowitz, M.D., Ph.D., Global Oncology Head, Janssen. “The rapid development and approval of DARZALEX – the first human anti-CD38 monoclonal antibody – is a great example of this commitment and our ongoing work in developing immunotherapies. We will continue to study this compound as both a mono- and a combination therapy to understand its full clinical benefit for patients across the treatment continuum in multiple myeloma and other tumor types.”
The warnings and precautions for DARZALEX include infusion reactions, interference with serological testing and interference with determination of complete response (see Important Safety Information).1 The most frequently reported adverse reactions (incidence ≥20%) were: fatigue, nausea, back pain, pyrexia, cough and upper respiratory tract infection.1
In data from three pooled clinical studies including a total of 156 patients, four percent of patients discontinued treatment due to adverse reactions.1 Infusion reactions were reported in approximately half of all patients treated with DARZALEX.1 Common (≥5 percent) symptoms of infusion reactions included nasal congestion, chills, cough, allergic rhinitis, throat irritation, dyspnea (shortness of breath) and nausea.1 Severe infusion reactions, including bronchospasm, dyspnea, hypoxia and hypertension (<2 percent each).1
The recommended dose of DARZALEX is 16 mg/kg body weight administered as an intravenous infusion.1 The dosing schedule begins with weekly administration (weeks 1-8) and reduces in frequency over time to every two weeks (weeks 9-24) and ultimately every four weeks (week 25 onwards until disease progression).1
In August 2012, Janssen Biotech, Inc. and Genmab A/S entered a worldwide agreement, which granted Janssen an exclusive license to develop, manufacture and commercialize DARZALEX.13 Janssen is currently the global sponsor of all but one clinical study. DARZALEX will be commercialized in the U.S. by Janssen Biotech, Inc.
About Multiple Myeloma
Multiple myeloma is an incurable blood cancer that occurs when malignant plasma cells grow uncontrollably in the bone marrow.2,3 Multiple myeloma is the third most common blood cancer in the U.S., following only leukemia and lymphoma.14 Approximately 26,850 new patients will be diagnosed with multiple myeloma, and approximately 11,240 people will die from the disease in the U.S. in 2015.15 Globally, it is estimated that 124,225 people will be diagnosed, and 87,084 will die from the disease in 2015.16,17 While some patients with multiple myeloma have no symptoms at all, most patients are diagnosed due to symptoms which can include bone problems, low blood counts, calcium elevation, kidney problems or infections.18 Patients who relapse after treatment with standard therapies (including PIs or immunomodulatory agents) typically have poor prognoses and few remaining options.3
Access to DARZALEX® (daratumumab) Injection, for Intravenous Infusion
DARZALEX (daratumumab) injection for intravenous infusion will be available for distribution in the U.S. within two weeks following FDA approval. Janssen Biotech offers comprehensive access and support information, resources and services to assist U.S. patients in gaining access to DARZALEX through the Janssen CarePath Program. For more information, health care providers or patients can contact: 1-844-55DARZA (1-844-553-2792). Information will also be available at www.DARZALEX.com. Dedicated case coordinators are available to work with both healthcare providers and patients.
Patients with private or commercial insurance may be eligible for the Janssen CarePath Savings Program for DARZALEX. Information on the enrollment process will be available online at www.darzalex.com/access-and-cost-support#affordability.
About DARZALEX® (daratumumab) Injection, for Intravenous Infusion
DARZALEX® (daratumumab) injection for intravenous infusion is indicated for the treatment of patients with multiple myeloma who have received at least three prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent, or who are double-refractory to a PI and an immunomodulatory agent.1 This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. DARZALEX is the first human anti-CD38 monoclonal antibody (mAb) to receive U.S. Food and Drug Administration (FDA) approval to treat multiple myeloma. DARZALEX is believed to induce tumor cell death through apoptosis, in which a series of molecular steps in a cell lead to its death1,10 and multiple immune-mediated mechanisms of action, including complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).1,8 More information will be available atwww.DARZALEX.com.

References
- World Health Organization (2009). “International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 101” (PDF). WHO Drug Information 23 (2).
- “‘Janssen Biotech Announces Global License and Development Agreement for Investigational Anti-Cancer Agent Daratumumab'”. Janssen Biotech. Retrieved 2013-01-31.
- “ASCO: Drug Shows Promise in Myeloma”. MedPage Today.
- “‘Daratumumab Continues To Show Promise For Relapsed/Refractory Myeloma Patients (ASH 2012)'”. The Myeloma Beacon. Retrieved 2013-01-31.
- Lokhorst, Henk M.; Plesner, Torben; Laubach, Jacob P.; Nahi, Hareth; Gimsing, Peter; Hansson, Markus; Minnema, Monique C.; Lassen, Ulrik; Krejcik, Jakub (2015-09-24). “Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma”. The New England Journal of Medicine 373 (13): 1207–1219. doi:10.1056/NEJMoa1506348. ISSN 1533-4406. PMID 26308596.
- http://www.medscape.com/viewarticle/854548?nlid=91686_3663&src=wnl_edit_newsal&uac=78316PX&impID=890536&faf=1
- Chapuy, CI; Nicholson, RT; Aguad, MD; Chapuy, B; Laubach, JP; Richardson, PG; Doshi, P; Kaufman, RM (June 2015). “Resolving the daratumumab interference with blood compatibility testing.”. Transfusion 55 (6 Pt 2): 1545–54. PMID 25764134.
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Human |
| Target | CD38 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | 945721-28-8 |
| ATC code | none |
| ChemSpider | none |
| UNII | 4Z63YK6E0E |
| Chemical data | |
| Formula | C6466H9996N1724O2010S42 |
| Molar mass | 145,391.67 g·mol−1 |
////Daratumumab