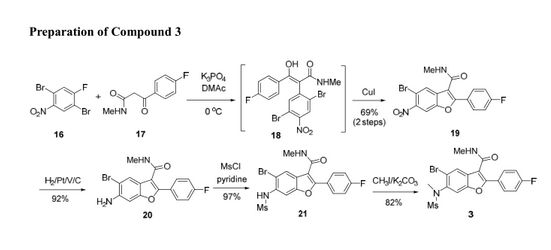
A novel and convergent approach to tetrasubstituted benzofurans was developed from ortho-bromo aryl fluorides and keto-amides via one-pot SNAr displacement and subsequent Cu(I) catalyzed C–O coupling on the ortho-bromide. The scope of this methodology was demonstrated on several similar substrates.
Concise Cu (I) Catalyzed Synthesis of Substituted Benzofurans via a Tandem SNAr/C–O Coupling Process
1 as a pale yellow solid (3.1 kg, 86% yield, 98.8% LACP). Mp: > 240 °C.
1H NMR (400 MHz, DMSO-d6)δ 8.54 (d, J = 4.5 Hz, 1H), 8.07 (s, 1H), 8.07–7.94 (m, 3H), 7.42 (t, J = 8.9 Hz, 2H), 3.34 (s, 3H), 3.22 (d, J = 4.1 Hz, 3H), 2.85 (d, J = 4.6 Hz, 3H);13C NMR (100 MHz, DMSO-d6) δ 26.2, 38.2, 112.8, 113.4, 115.9 (d, J = 22 Hz), 119.7, 124.2, 125.2, 128.7, 129.6 (d, J = 8.8 Hz), 136.9, 151.8, 154.4, 162.4, 162.9 (d, J = 247.1 Hz).
19F NMR (376 MHz DMSO-d6) δ 109.9
AHR-FAB-MS calcd for C18H16BrFN2O4S: MH+, 455.2980. Found: 455.0055 (MH+).
-
(a) Burns, C. J., Del Vecchio, A. M., Bailey, T. R., Kulkarni, B. A., Faitg, T. H., Sherk, S. R., Black-Ledge,C. W., Rys, D. J., Lessen, T. A., Swestock, J., Deng, Y., Nitz, Theodore, J., Reinardt, J. A., Feng, H., andSaha, A. K. Patent WO 2004041201.
(b) McComas, C. C., Liverton, N. J., Habermann, J., Koch, U.,Narjes, F., Li, P., Peng, X., Soll, R., and Wu, H. WO 2011106929.
(c) McComas, C. C., Liverton, N. J., Soll,R., Li, P., Peng, X., and Wu, H. WO 2011106986.
(d) McComas, C. C., Liverton, N. J., Soll, R., Li, P.,Peng, X., Wu, H., Narjes, F., Habermann, J., Koch, U., and Liu, S. WO 2011106992.
(e) McComas, C. C.,Liverton, N. J., Habermann, J., Koch, U., Narjes, F., Li, P., Peng, X., Soll, R., Wu, H., Palani, A., He, S.,Dai, X., Liu, H., Lai, Z., London, C., Xiao, D., zorn, N., and Nargund, R. WO 2013033971.
-
He, S.; Li, P.; Dai, X.; McComas, C. C.; Du, C.; Wang, P.; Lai, Z.; Liu, H.; Yin, J.; Bulger, P. G.; Dang, Q.;Xiao, D.; Zorn, N.; Peng, X.; Nargund, R. P.; Palani, A. Tetrahedron Lett. 2014, 55, 2212– 2216, DOI: 10.1016/j.tetlet.2014.02.051
//////Concise Cu (I), Catalyzed, Synthesis, Substituted Benzofurans, Tandem SNAr/C–O Coupling Process